La Vague 53
Qualification approach for the validation of real-word shipping in single-use systems
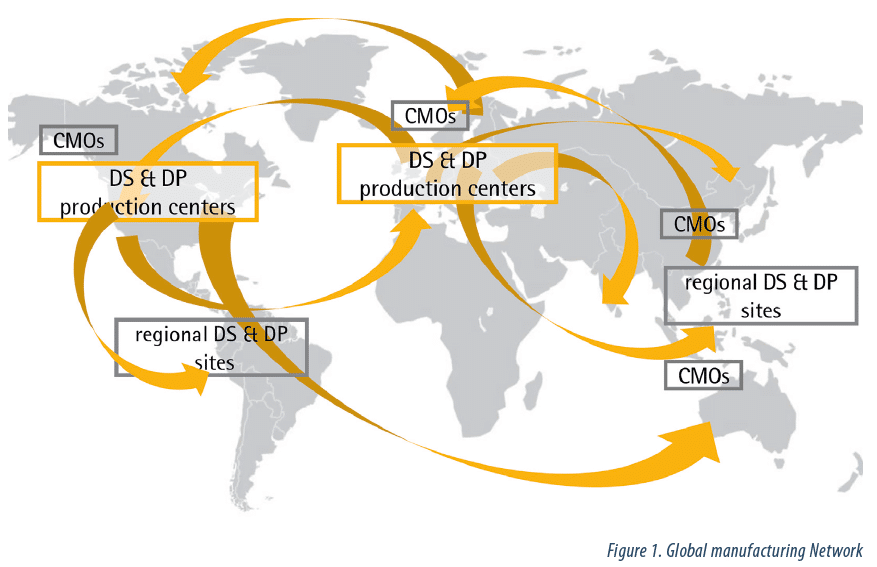
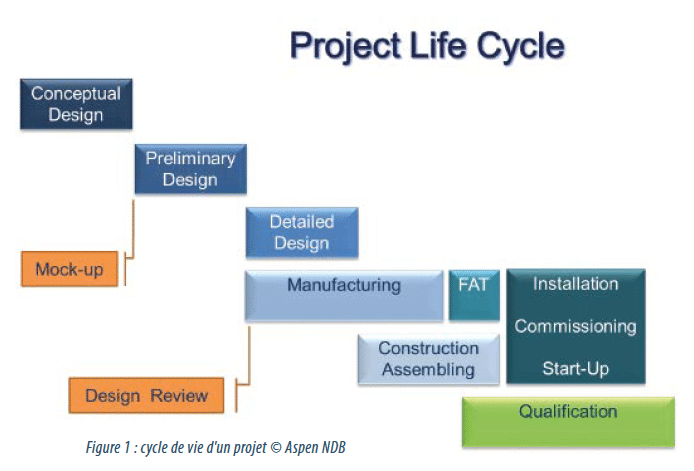
The complexity of biopharmaceutical manufacturing processes requires continuous improvement. As shown in figure 1, the expansion of manufacturing capacity worldwide has resulted in the multiplication of links between production facilities as well as the increasing need for storage or transportation of media, intermediate, BDS, and drug products between sites over the world.
Outsourcing to contract manufacturing organizations (CMOs) offers a solution to the capacity constraints and reduces the total risks associated with building internal capacity; however, a robust and validated manufacturing process (2), including product transportation between facilities, is then required.