La Vague 54
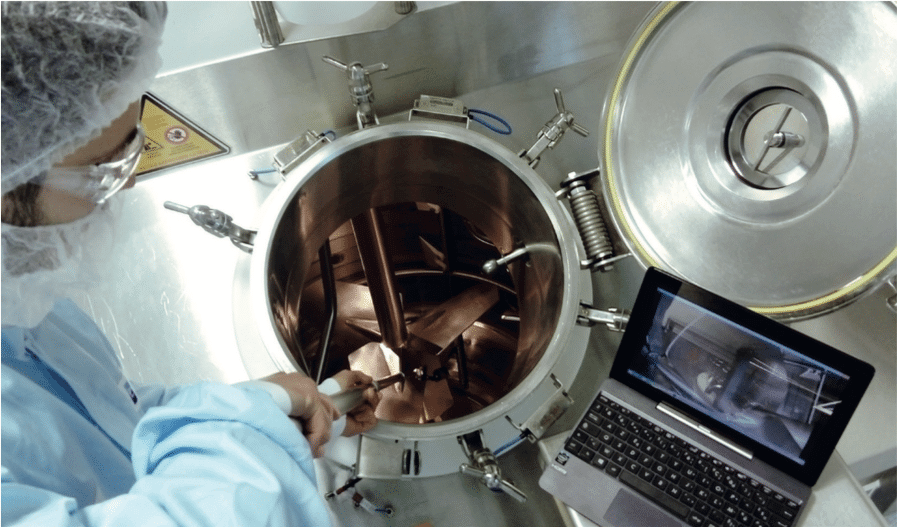
Le contrôle visuel indirect modernise la validation du nettoyage
La Vague 54
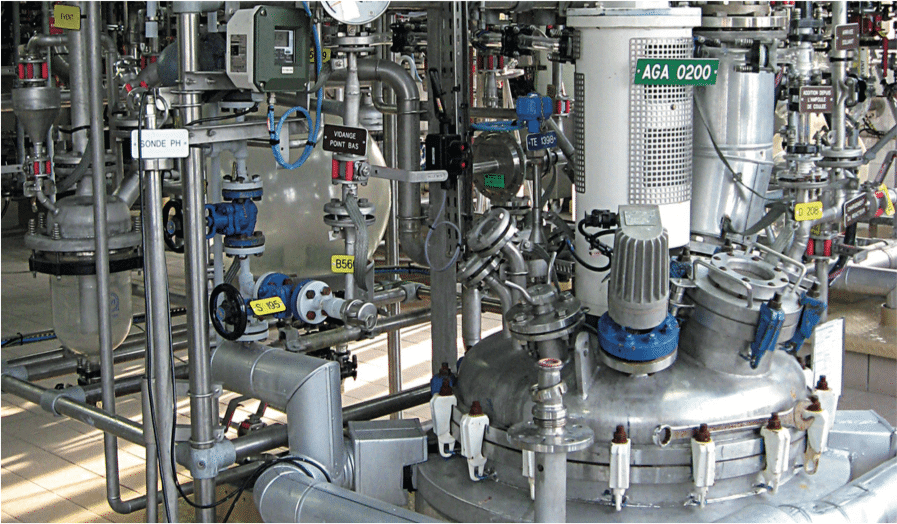
La détermination du taux de recouvrement est une étape préliminaire, nécessaire lors d’un exercice de validation du nettoyage
La Vague 54
Cleaning Validation for biotechnological substances : What acceptance criteria ?
La Vague 54
“Health-based approach” implementation for setting limits in cleaning validation for Vaccines/Biotech
La Vague 54
Le GIC A3P Validation du Nettoyage prépare un guide pratique pour l’automne 2017 sur le Chapitre 10 de l’Annexe 15 des GMP Européennes : Présentation & discussion.
La Vague 53
Low Endotoxin Recovery (LER) is today one of authorities serious concerns regarding pyrogen testing
Lipopolysaccharides / endotoxins are a subtype of pyrogens originating from the cell wall of gram-negative bacteria that are both extremely thermostable and very potent when brought into contact with the human immune system. But they are by no means the only possible pyrogenic contamination – and the growing complexity of biotechnological products increased the risk of having pyrogens present that originate from a variety of microorganisms and other sources.
La Vague 53
Single Use & Stainless Steel: complementarity or fight?
There has been a huge increase in single use apparatus in the biopharmaceutical manufacturing world during the last. Many companies compete in manufacturing production tools. At Boccard where our mantra is “In Stainless Steel We Trust”, we have a different opinion. We believe that plastic and stainless steel are complementary. Let’s take the example of cell culture. Before growing in a huge fermenter (e.g. 1000 L), what is the point of using a stainless steel 20 L fermenter as a first step from an economical point of view? why not use Single Use apparatus in this case?
La Vague 53
Expansion of Human Bone Marrow-Derived Mesenchymal Stem Cells in BioBLU 0.3c Single-Use Bioreactors
Mesenchymal stem cells (MSCs) are attractive candidates for therapeutic applications, especially in the field of regenerative medicine [1] because – in contrast to embryonic stem cells – they do not pose ethical issues, they can be isolated from various tissue sources, and they reduce the risk of rejection reactions. The doses of human MSCs (hMSCs) needed for clinical trials are estimated at between one and 200 million cells per patient, depending on the disease being tackled [2]. One of the most important challenges in providing hMSCs for curative use is the production of large quantities of cells in a robust manner.
La Vague 53
Qualification approach for the validation of real-word shipping in single-use systems
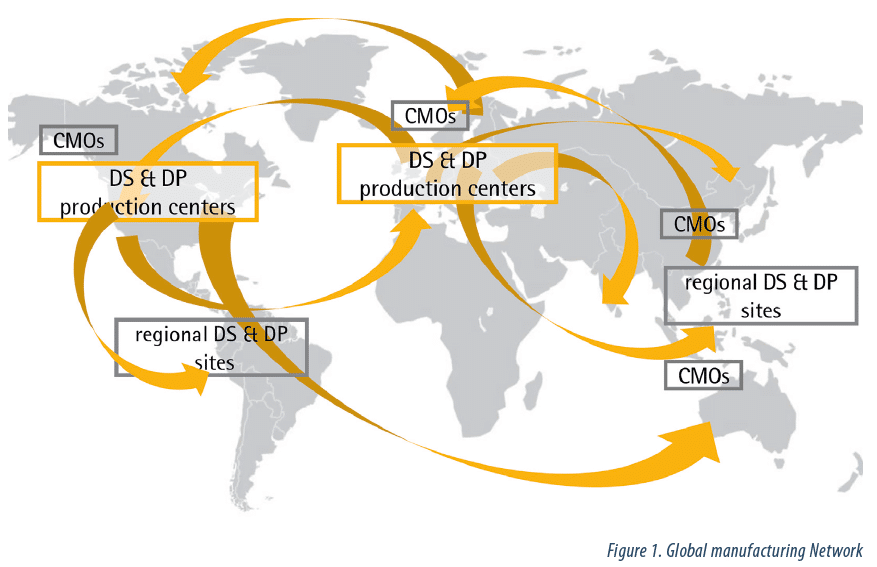
The complexity of biopharmaceutical manufacturing processes requires continuous improvement. As shown in figure 1, the expansion of manufacturing capacity worldwide has resulted in the multiplication of links between production facilities as well as the increasing need for storage or transportation of media, intermediate, BDS, and drug products between sites over the world.
Outsourcing to contract manufacturing organizations (CMOs) offers a solution to the capacity constraints and reduces the total risks associated with building internal capacity; however, a robust and validated manufacturing process (2), including product transportation between facilities, is then required.