Summary
- There is everything to gain from working in campaign mode, a breakthrough in isolator productivity
- Visual inspection: main findings of ANSM inspections
- Cleaning validation of production equipment: Visual inspection, accreditation of staff in “visually clean”
- A Refresher on Disinfectant Wet Contact Time
- THE EU MDR and IVDR : Combination products now subject to the same degree of surveillance as standalone medical devices
- Suitability of the Mono-Mac-6 cell line for the detection of endotoxins and non-endotoxin pyrogens
- Biological indicators, random growth. Is your decontamination cycle really at fault?
- Determining a Strategy for Container Closure Integrity Testing of Sterile Injectable Products
- Efficient Control Strategy enabled by structured Knowledge
- High-performance solution for real-time colony counts on filtration membranes in microbiological analysis with ScanStation
Cleaning validation of production equipment: Visual inspection, accreditation of staff in “visually clean”
The cleaning of production equipment is a fundamental aspect of GMPs to avoid all cross contamination of pharmaceutical products during their manufacture. Validation of cleaning processes has been required for a long time in the cGMP industries and is recognised as an important activity for control of the risk of product contamination so ensuring their quality but above all ensuring patient safety.

Cleaning validations aim to prove that cleaning methods are effective, reliable, robust and capable of removing contamination up to a set level.
A visual inspection of equipment is required by the regulations after the cleaning steps as well as before the start of production. Visual inspection forms part of routine cleaning and represents the first criterion in cleaning validation. The results of this inspection must be judged acceptable before taking samples by swabbing or by other methods from the cleaned equipment for the purposes of analytical confirmation.
Since the 1990s, several publications (1),(2),(3),(4),(5),(6) have addressed the theme of the study of visual inspection, stating that the limit of detection is thought to be located in a concentration range extending from around 1 µg/cm² to more than 10 µg/cm² depending on the products investigated and inspection conditions. US regulations (7) require “an inspection of production equipment immediately before use”. The PIC/S (8) advises carrying out “an investigation of surface contamination in order to determine the concentration at which most ingredients are visible”. The latest EMA text Q&A8 (9) specifies that within the context of cleaning inspection after validation and in certain specific cases “staff carrying out visual inspections must receive specific training and regular tests of visual acuity. And that accreditation must be proven by practical trials”.
It is therefore important to take account of this technique and it is essential to be able to provide justification that these tests are carried out under the right conditions. It must be supervised by an appropriate quality system.
Visual inspection must be carried out on the surfaces of equipment in direct and indirect contact with the product and requires that these surfaces are visually accessible. In the event that surfaces are not visually accessible, it is recommended that the equipment be disassembled to access it or to use light sources, mirrors or endoscopes. The residues or defects looked for may be from different sources: previous products, cleaning agents, microbial contamination, equipment wear (e.g. scratches, rouging, etc.), foreign materials, technical liquids (such as oil or grease), rinsing solvent residues (e.g. residual water), etc.
Routinely, this visual inspection is carried out on equipment by many people during or at the end of use, after automatic cleaning (clean in place), semi-automatic or manual cleaning, on large surfaces or on small parts cleaned in a washer. These inspections are usually tracked in checklists left on the equipment until use, then attached to the batch record and to site internal quality documents in order to justify that the equipment is clean and usable for upcoming production. Teams also carry out a visual inspection of the equipment at the start of production. This information is also tracked in batch records and in equipment or production area logbooks. Most often, an initial check is carried out when cleaning is finished by one person and just before the equipment is reused by a second person different from the first. The quality and qualification/validation teams also inspect the equipment regularly as part of their activities. Very often, the training and accreditation of these individuals in visual inspection is not tracked in an appropriate manner, nor considered a critical activity during accreditations and often forms part of global training and accreditation at the workstation.
Likewise, as part of the process of putting cleaning validations in place, the samplers who take swabs, samples of rinsing water or microbiological samples are specifically trained and accredited in an official way (e.g. laboratory-based recovery tests). Accreditation in visual inspection should then be referenced and carried out in the same way as other accreditation processes performed on the site and considered as being equally important.
This article details one of the approaches possible for the implementation, management and monitoring of the accreditation of teams in visual inspection in the context of cleaning validations and routine inspection of equipment. It describes the broad lines of an approach which can be adapted, optimised and modified so as to enable its application to the internal procedures of each site. This accreditation cannot justify the fact that only visual inspection is taken into account when ruling on the compliance of cleaning. This accreditation proves that staff that have undergone training will be capable of visually detecting surface contamination below the acceptable contamination limit, of defining the clean status of equipment and in this way of confirming that the risk of cross contamination of equipment is controlled.
Qualification of the principles of visual inspection must be differentiated from staff accreditation. The accreditation of teams is the final step in the establishment of the overall visual inspection strategy. It will be necessary to determine beforehand the acceptance limits for residues on equipment surfaces, then to determine the visual detection limit before being able to put in place the process for accreditation of teams. The qualification of visual inspection principles should be validated before carrying out team accreditation trials (10).
Implementation of accreditation in visual inspection requires transversal organisation. It is carried out on small groups of individuals (production operators, samplers, etc.) under the responsibility of the Quality Assurance Department. The test is usually developed by the laboratory which has the resources to implement these tests successfully (products, solvents, coupons made from different materials, etc.).
Routinely, visual inspection is different when the inspection is performed on small parts which can be placed in ideal test conditions (angle, lighting, distance) unlike inspections carried out on large items of fixed equipment for which the inspection parameters will be more restrictive (fixed angle and non-optimal light conditions, low light levels inside tubing or large containers and distanced from surfaces). In order to homogenise the approach to qualification and accreditation, tests are carried out on coupons of defined size (e.g.: coupon of 100 cm²), of a material and with a surface quality as close as possible to the materials encountered in production. Tests are conventionally carried out with stainless steel, glass and teflon coupons.
The visual inspection acceptance criteria must be defined for all contaminants potentially present on equipment: residues of active substances, finished products or cleaning agents. It is not necessary to carry out “visually clean” tests for microbial contamination as the latter is closely linked to the residues of other products which might remain on the surfaces.
1. Determination of the visible residue limit (VRL)
The first step in the visual inspection strategy is the definition of the visible residue limit, that is the smallest concentration of product which can be detected by human eye on the surface of a material. The marker to be used preferentially for these tests is the product derived from the “worst case” risk analysis detailed in the cleaning validations strategy (e.g.: cleaning validation master plan), that is to say the raw material, active substance, finished product or residue that is the hardest to clean from each defined product family. If it is easier to use the finished product that contains the active substance that is most difficult to clean, then this finished product can be used for tests and vice versa. The choice of contaminant used to carry out the study must be justified.
Several concentrations must be studied. In the first instance it is necessary to determine the acceptable surface limit: (ASL which is evaluated in µg /cm2) according to the toxicological and therapeutic approaches of the products manufactured. These acceptance criteria are normally defined when cleaning validations are being established with the value being calculated in accordance with the recommendations of the cleaning validation master plan.
Secondly, the visual residue limit VRL (in µg/cm2) must be defined. It is recommended that various dilutions of the ASL concentration be deposited on coupons of different materials up to a very weak concentration (e.g.: several successive dilutions by a factor of 10, or 2). These concentrations are to be defined and adjusted by the team implementing the test on site. Enough coupons must be used to provide proper representation of the concentration range under investigation: around ten plates is appropriate as a minimum.
The deposit is produced preferably with a spray so that it is distributed homogeneously on the coupon surface and between coupons. Deposits produced using a pipette may cause heaping of the product which is difficult to repeat from one deposit to the next. The deposits must be dry and produced as close as possible in time to the viewing tests. A study of the stability of the visual appearance of residues on surfaces can be performed beforehand in order to be able to keep the coupons overloaded for a defined time after deposit.
It is recommended that the test be conducted in light conditions close to those encountered in the production area. Light levels can be noted in different production areas and a range of light levels can be taken into account in conducting qualification and accreditation tests.
The coupons can be arranged in order of decreasing concentration on a flat surface, with light levels close to those of production areas. Note down and transfer the light level associated with each coupon onto the result sheets before the test.
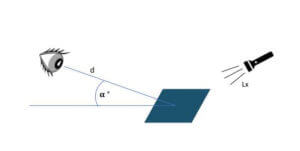
An adequate number of people (as many as possible) should inspect the coupons in order to obtain representative results. It is possible to involve individuals from different departments, of different ages, of different levels of seniority within the company and those who wear or who do not wear corrective lenses in order to have the widest range of people possible. Staff should not be specially trained in visual inspection to take this test. Viewing should take place under conditions close to those in production areas.
The important parameters to consider are:
- viewing distance (d);
- angle of view (a);
- light (Lx).
These viewing parameters must as far as possible be kept identical for all people carrying out the test.
It is recommended that VRL determination tests are performed several times (for example 3 times) and if possible on different days in order to obtain a more robust representation of results.
The visual residue limit concentration (VRL) is established as being the lowest concentration detected by 100% of operators. Comparison of the VRL concentration with the ASL concentration enables determination of whether the residues acceptance limit will be detectable on equipment surfaces by staff carrying out the inspections.
- In the event that the VRL concentration > ASL, it will be difficult for staff carrying out visual inspections to detect the acceptable contamination limit on production surfaces. In this case, it is absolutely necessary to supplement visual inspection with analytical methods that have appropriate detection capacity relative to the ASL limit.
- In the event that the VRL concentration is close to the ASL concentration, it is still recommended that visual inspection be supplemented by analytical testing with appropriate detection capacity.
- In the event that the VRL concentration £ ASL (ideally beyond a factor of 100) (11), visual inspection by the staff will be sensitive enough to detect the acceptable contamination limit on surfaces. Accreditation of teams can then be carried out against these parameters.
2. Accreditation of staff
The aim of this accreditation is not to disqualify staff who do not pass the test (operational discrimination) but to provide adequate training so that everyone is capable of inspecting surfaces after cleaning and having effective control over the state of cleanliness of surfaces.
Before conducting accreditation tests, staff must take appropriate training in visual inspection. This may be conducted in the following manner:
- Reading the procedures that describe the performance of visual inspections,
- Making staff aware of the types of residue that may potentially be present on surfaces after cleaning and which must be looked for during inspections,
- Familiarisation of staff with equipment. That is becoming familiar with the equipment surfaces that will be inspected, and knowing the surfaces that are difficult to access or the surfaces that require use of an inspection aid device (endoscope, mirror, etc.),
- Familiarisation of staff with the tests that will be conducted during accreditation and the different concentrations investigated (VRL, ASL),
- Viewing soiled control coupons to obtain experience relevant to the accreditation tests.
The procedures that describe visual inspection must be as detailed as possible, and may include photos, diagrams, accessories and inspection position and be comprehensible in order to ensure reproducibility of inspections.
Coupons must be soiled using the same product, in the same way as for definition of the VRL concentration. The deposits must be homogeneous and dry for the performance of tests.
Concentrations must be adapted for the purposes of conducting the accreditation test relative to the ASL acceptance limit.
It is recommended that tests be conducted with coupons soiled at the limit concentration (ASL) and with other coupons soiled with another concentration which will be between the ASL and the visual residue limit (VRL). Example: 50% of the ASL or (VRL + ASL)/2.
An adequate number of coupons must be used in order to obtain representative results (as a minimum 10 coupons for each concentration). Blank coupons are prepared by depositing a quantity of pure solvent (corresponding to the quantity of solvent contained in the ASL concentration solution (example: purified water or WFI water).
Coupons must be identified in such a way that the person performing the test cannot detect coupon status (clean / dirty) and they must be deposited on a flat surface. For accreditation purposes, they must be deposited randomly. The light level associated with each coupon must be recorded with a light meter. Each person taking part in the accreditation must check each coupon one after the other under the test conditions defined in the associated procedures (angle, light level, distance) and transfer the status of each coupon inspected onto a test sheet: “dirty” or “clean”. Only 2 entries (dirty /clean) are accepted. It is not necessary to determine whether some coupons are more or less dirty than others. The coupons soiled at the ASL concentration should normally be identified as dirty. Coupons soiled at the concentration ASL/2 should also be identified as dirty if this concentration is higher than the VRL. Blank coupons must be identified as clean.
Each test sheet is then studied and the results are summarised by carrying forward:
- the percentage detection of dirty coupons compared to the actual number of dirty coupons,
- the percentage detection of clean coupons compared to the actual number of clean coupons.
3. Acceptance criteria
The results expected following staff accreditation tests:
All loaded coupons are detected as dirty: 100% of coupons considered dirty. All blank coupons are detected as clean: 100% of coupons considered clean.
The term clean is defined as an absence of visually detectable residue.
- If a clean coupon is identified as dirty, that should not be considered an accreditation failure. This error may be caused by caution or by a slight defect in the surface of the coupon in question and these errors may be accepted. If several errors (number to be defined on each site) of this type are committed by the accredited person, it is important to carry out an awareness-raising exercise before confirming the accreditation.
- If a dirty coupon is identified as clean, then the accreditation can be considered a failure because visually dirty equipment could be considered clean and released for subsequent production. The equipment could be used to manufacture a different product and could be the cause of cross contamination between productions. In this specific case, the individual must be subject to visual inspection training, which may be conducted via the inspection of coupons used for accreditation at a distance closer than that used in the test. This awareness-raising exercise can also involve rereading the inspection procedure and/or individual training in the inspection procedure. The test can be repeated in order to confirm accreditation of the individual. Each site must assess whether the individual is capable of carrying out a visual inspection and manage this accreditation in the most ethical way possible.
4. Monitoring of accreditation
Following accreditation of teams, it is necessary to check whether any drift appears in results during visual inspections on a regular basis. For this, it is recommended that teams be audited regularly while carrying out inspections and that they take part in visual inspection tests using coupons as described in the previous paragraph. Testing frequency depends on the practices of each site. If only visual inspection is used for cleaning checks after validation of cleaning procedures, then staff must take a visual acuity test regularly (EMA Q&A8) (9).
Conclusion
The visual inspection accreditation strategy in the context of cleaning validations or routine cleaning inspections is a very important point to consider and is very often forgotten or implemented in a very condensed even approximate manner. Several scientific groups are currently working on developing a complete accreditation procedure, taking account of statistical studies and expanded tests to define accreditation techniques. This article has presented one of the possibilities for the implementation, justification and supervision of visual inspection in a simple and rapid manner.
According to the latest EMA text Q&A8 (9) in some specific cases, visual inspection could be used alone to rule on the cleanliness of equipment routinely after cleaning validation (EMA text Q&A7) (9). In this case, the setting up of a robust and effective visual accreditation strategy should be implemented and will be expected by the health authorities.
Partager l’article

Christophe Gamblin – Theraxel
Titulaire d’un master scientifique et après plus de 15 ans d’expérience dans l’industrie pharmaceutique en tant que responsable contrôle qualité analytique avec une spécialisation dans la validation des nettoyages, Christophe est maintenant un expert qui accompagne les industriels dans la maitrise de la contamination croisée. Membre de l’organisation ASTM, il participe à l’élaboration de nouvelles normes scientifiques sur le risque chimique dans l’industrie pharmaceutique et biotechnologie.
christophe.gamblin@theraxel.fr
Glossaire
GMP: Good Manufacturing Practices
cGMP: Current Good Manufacturing Practices
LAS : Limit Acceptance Surface (µg/cm2)
VRL : Visual Residual Limit (µg/cm2)
Références
1. Determining Cleaning Validation Acceptance Limits for Pharmaceutical Manufacturing Operations. Fourman, G. L. and Mullen, M. V. 4, 1993, Pharmaceutical Technology, Vol. 17, pp. 54-60.
2. Bestimmung der Sichtbarkeitsgrenzen von pharmazeutischen Feststoffen auf Edelstahloberflächen. Buscalferri, Frank, Lorenzen, Sabine, Schmidt, Michael, Schwarm, Hans-Martin, Anhalt, Ehrhard, et al. 6, 2000, Pharm. Ind., Vol. 62.
3. Cleaning Validation: An Overall Perspective. Jenkins, K. M. and Vander Weilen, A. J. 4, 1994, Pharmaceutical Technology, Vol. 18, pp. 60-73.
4. Visible-Residue Limit for Cleaning Validation and its Potential Application in Pharmaceutical Research Facility. Forsyth, Richard J., Van Nostrand, Vincent, and Martin, Gregory P. 10, 2004, Pharmaceutical Technology, Vol. 28.
5. Using Visible Residue Limits for Introducing New Compounds into a Pharmaceutical Research Facility. Forsyth, Richard J. and Van Nostrand, Vincent. 10, 2005, Pharmaceutical Technology, Vol. 29.
6. Validation of Visual Inspection as an Analytical Method for Cleaning Validation. Desai, Parth and Walsh, Andrew. 2017, Pharmaceutical Online.
7. U.S. Food and Drug Administration. 21 CFR 211.67 (b).
8. Pharmaceutical Inspection Co-operation Scheme (PIC/S).
9. European Medecines Agency (EMA). Questions and answers on implementation of risk-based prevention of cross-contamination in production and ‘Guideline on setting health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facili. 2018. EMA/CHMP/CVMP/SWP/246844/2018.
10. Justification and Qualification of Visual Inspection for use in Cleaning Validation for a Low Risk, Multi-Product Facility. Walsh, Andrew, Liu, Dongni (Nina), and Ovais, Mohammad. August 2018, Pharmaceutical Online.
11. Walsh, Andrew, et al. An MSSR-Derived Scale For Assessing Detectability Of Visual Inspection.
12. ASTM International. Standard Guide for Science-Based and Risk-Based Cleaning Process Development and Validation. [Online] 2018. doi: https://doi.org/10.1520/E3106-18E01. doi: https://doi.org/10.1520/E3106-18E01.
13. Agency, European Medicines. Questions and answers on implementation of risk-based prevention of cross-contamination in production and ‘Guideline on setting HBEL for use in risk identification in the manufacture of different medicinal products in shared facilities. 2018.