Summary
- There is everything to gain from working in campaign mode, a breakthrough in isolator productivity
- Visual inspection: main findings of ANSM inspections
- Cleaning validation of production equipment: Visual inspection, accreditation of staff in “visually clean”
- A Refresher on Disinfectant Wet Contact Time
- THE EU MDR and IVDR : Combination products now subject to the same degree of surveillance as standalone medical devices
- Suitability of the Mono-Mac-6 cell line for the detection of endotoxins and non-endotoxin pyrogens
- Biological indicators, random growth. Is your decontamination cycle really at fault?
- Determining a Strategy for Container Closure Integrity Testing of Sterile Injectable Products
- Efficient Control Strategy enabled by structured Knowledge
- High-performance solution for real-time colony counts on filtration membranes in microbiological analysis with ScanStation
Suitability of the Mono-Mac-6 cell line for the detection of endotoxins and non-endotoxin pyrogens
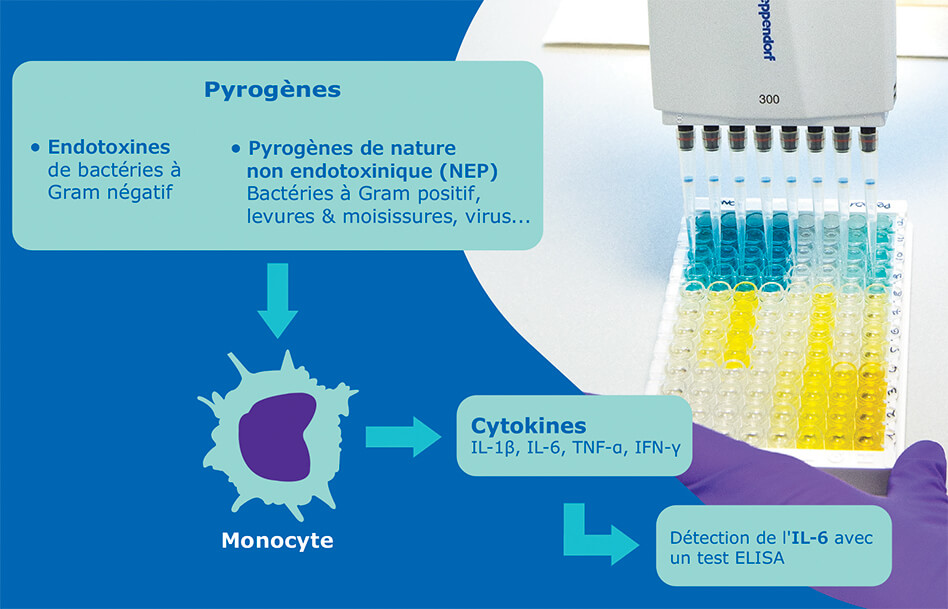
Pyrogen detection is one of the mandatory release tests for sterile parenteral drugs. Today, the two main test methods in use are the bacterial endotoxin test (or LAL test) and the Rabbit Pyrogen Test.
Another test method that has increasingly been used the past few years does not need animals for pyrogen detection, in line with current European regulatory guidelines. Indeed, the monocyte activation test (MAT) uses human monocytic cells to mimic the human reaction to pyrogens in vitro.
Yet, the MAT remains little known. Its adoption rate is growing only slowly despite having been described as a compendial method in the European Pharmacopeia since 2010 (chapter 2.6.30). Recently, several test solutions have been commercialized, allowing an easy adoption and use in the lab.
The various available kits use different sources of monocytes. The solutions are based either on cryopreserved whole blood, peripheral blood mononuclear cells (PBMC) or monocytes from the Mono-Mac-6 cell line (MM6).
The MM6 cell line, which has been validated for the use in the MAT, has sometimes been questioned over its ability to detect non-endotoxin pyrogens (NEPs). To demonstrate its performance, tests have been performed using a wide panel of toll-like receptor (TLR) ligands. TLRs are monocytic receptors responsible for the detection of pyrogens. This article gives an overview of the results obtained.
1. Mode of action of pyrogens
Pyrogens trigger fever through the activation of the innate immune system, and more specifically through the activation of monocytes. These white blood cells recognize antigens thanks to cell-surface receptors called pattern recognition receptors (PRRs). Activation of PRRs leads to the production of endogenous pyrogens such as cytokines, which have a direct effect on temperature regulation in the hypothalamus.
Pyrogens from various sources (bacteria, viruses, yeasts and molds) are recognized by specific types of PRR called toll-like receptors (TLRs), which are expressed by monocytes. Bacterial cell wall components are broadly recognized by cell surface TLRs, whereas nucleic acids are recognized by intracellular TLRs.
The diversity of the TLR family and the specificity of individual TLRs for the detection of different ligands support the hypothesis that the human fever reaction can be provoked not only by lipopolysaccharides (LPS), but also by many other substances originating from both Gram-negative and Gram-positive bacteria, fungi, yeasts, viruses, and parasites.
Pyrogens are often categorized into two main groups: endotoxins (LPS from Gram-negative bacteria) and non-endotoxin pyrogens (NEPs).
2. Detection of Pyrogens
Pyrogenic substances in pharmaceutical products can induce life-threatening fever reactions after injection into the human body. Therefore, it is a regulatory requirement to test such products for pyrogens to ensure product quality and patient safety.
Today, several methods are available for pyrogen detection in pharmaceutical samples. The most commonly used method remains the bacterial endotoxin test (BET) despite its specificity for endotoxin only, a specific type of pyrogen originating from Gram negative bacteria and targeting the TLR4 of monocytic cells.
To cover a broader range of pyrogenic contaminants, the RPT can be used. However, its low robustness and the need for animals to perform the test bring some strong drawbacks to this method.
The MAT remains the only method to evaluate the pyrogenicity of a sample through an in vitro test using human monocytic cells. This test actually mimics what happens in the human body: in the presence of pyrogens, the monocytes are activated and produce cytokines such as interleukin-6, which is subsequently detected in an ELISA test.
To perform the MAT, several sources of human monocytes can be used: whole blood, primary blood mononuclear cells (PBMCs) or a monocytic cell line, but so far only the Mono-Mac-6 (MM6) cell line has been validated to be used in an MAT. All these monocytic cell sources are listed as valid in the European Pharmacopeia Chapter 2.6.30 (Monocyte activation test). However, using a cell line for the MAT has sometimes been challenged due to the lack of evidence that it can detect NEPs.
3. Suitability of the Mono-Mac-6 cell line for the detection of NEPs
Several tests have been performed to demonstrate the suitability of the MM6 cell line to detect ligands for various extracellular and intracellular TLRs. For this purpose, the PyroMAT® System was used.
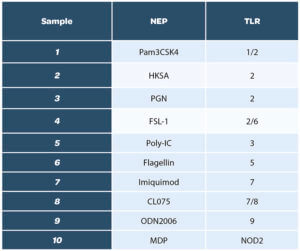
A total of ten ligands was evaluated, to cover the whole range of TLRs:
We decided to have a wide list of NEPs, including the ones mentioned in the European pharmacopeia Chapter 2.6.30, section 6-3
Prior to performing the study, the concentration of each ligand to be tested was determined and chosen so it would be within the quantification range of the test method. In addition, the ligands were tested for endotoxin contamination.
The different samples were used in a dose screening to evaluate the limits of detection in the monocyte activation test. For this, at least four dilutions of every NEP were run individually in the assay. An example of the results is shown below for the HKSA sample*:
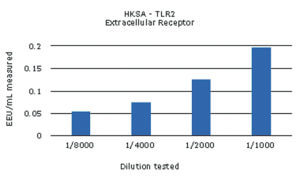
The non-endotoxin pyrogens tested were all detected, with values over the cut-off of the respective assay. The contaminations with MDP and ODN2006 could be detected but not quantified, as the calculated values were below the validated limit of quantification, 0.05 EU/mL.
In general, non-endotoxin pyrogens recognized by a cell surface receptor showed a dose-dependent increase of pyrogenicity in the monocyte activation test, but dose-dependency was less pronounced for pyrogens recognized by intracellular receptors. This indicates that the internalization process is an important factor in the reaction and that the quantification of non-endotoxin pyrogens with intracellular receptors might be hampered by the need for the cells to internalize the pyrogens.
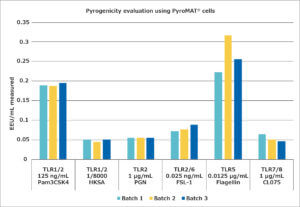
Following the assays to determine the suitable concentrations of the NEPs for the monocyte activation test, the stability of the reaction over several batches of PyroMAT® cells was evaluated. For this evaluation, only those pyrogens showing a dose-dependent response were used, as only for those would any differences in cell reactivity be expected to have a large influence on the quantification of the contamination. The results are shown below:
The different batches show the same reactivity for pyrogens, confirming the standardized reactivity of the Mono-Mac-6 cell line.
4. Potential synergistic effects of pyrogens in mixtures
Assays performed with single pyrogenic contaminants can be misleading, as contaminations in pharmaceutical products or on medical devices rarely contain just one type of pyrogen. Even in contaminations with a single microorganism species, several toll-like receptors can be engaged, targeting different cell wall components or bacterial structures (e.g. in the case of flagella-bearing bacteria).
A major advantage of the monocyte activation test is its ability to show the total response of the activated monocytes, resulting in an efficient evaluation of the pyrogenicity of a mixture of pyrogens in a human test system.
This was analyzed by adding endotoxin at the limit of detection of the assay to some of the above tested pyrogens at their respective limits of detection. The results reveal strong synergies for some pyrogens with cell-surface and intracellular receptors, except for imiquimod.
The effect was dependent on the dose of the endotoxin present among the non-endotoxin pyrogens. This led to a striking non-linearity of the result obtained using different dilutions of a sample contaminated with several pyrogenic entities. This highlights that the test should, wherever possible, be run with the lowest possible dilution (highest concentration) at which the sample does not interfere with the assay.
Conclusion
With this study, we have demonstrated that the Mono-Mac-6 cells used in the PyroMAT® system can detect a wide range of ligands targeting various TLRs, including intracellular ones. The MAT-based PyroMAT® system also shows a reproducible reaction to reference standard endotoxin and non-endotoxin pyrogens.
In addition, it is able to detect synergistic activation of multiple TLRs on the cell surface in presence of e.g. endotoxin and one other non-endotoxin pyrogen. The assay is therefore capable of detecting contaminations with individual pyrogens as well as mixtures and predicting the response of the human immune system to the contamination.
Partager l’article
Laure Robert – Merck
laure.robert@merckgroup.com
References
1. European Pharmacopoeia, chapter Monocyte activation test (2.6.30)
2. Akira, S.et al. 2006. Pathogen recognition and innate immunity. Cell 124, 783-801.
3. Beutler, B:A. (2009). TLRs and innate immunity. Blood 113, 1399-1407.
4. Kawai and Akira 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34, 637-650.
5. Henderson, B. and Wilson, M. 1996. Cytokine induction by bacteria: beyond lipopolysaccharide. Cytokine 8, 269-282.
6. Takeuchi, O. et al. Cutting edge: Role of Toll-like receptor 1in mediating immune response to microbial lipoproteins. J. Immunol. 169, 10–14 (2002).
7. Wyllie, D. H. et al. Evidence for an accessory protein function for Toll-like receptor 1 in anti-bacterial responses. J. Immunol. 165, 7125–7132 (2002).
8. Aliprantis, A. O. et al. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor 2. Science 285, 736–739 (1999).
9. Takeuchi, O. et al. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive cell wall components. Immunity 11, 443–451 (1999).
10. Schwadner, R. et al. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274, 17406–17409 (1999).
11. Schwadner, R. et al. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274, 17406–17409 (1999).
12. Means, T. K. et al. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J. Immunol. 163, 3920–3977 (1999).
13. Hajjar, A. M. et al. Cutting Edge: Functional interactions between Toll-like receptor (TLR) 2 and TLR1 or TLR6 in response to phenol-soluble modulin. J. Immunol. 166, 15–19 (2001).
14. Coelho, P. S. et al. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes induce in vivo leukocyte recruitment dependent on MCP-1 production by IFN-γ-primedmacrophages. J. Leukoc. Biol. 71, 837–844 (2002).
15. Opitz, B. et al. Toll-like receptor-2 mediates Treponema glycolipid and lipoteichoic acid-induced NF-κB translocation. J. Biol. Chem. 276, 22041–22047 (2001).
16. Massari, P. et al. Cutting edge: Immune stimulation by Neisserial porins is Toll-like receptor 2 and MyD88 dependent. J. Immunol. 168, 1533–1537 (2002).
17. Werts, C. et al. Leptospiral liopolysaccharide activates cells through a TLR2-dependent mechanism. Nature Immunol. 2, 346–352 (2001).
18. Hirschfeld, M. et al. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69, 1477–1482 (2001).
19. Underhill D. M. et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 (1999).
20. Asea, A. et al. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277, 15028–15034 (2002).
21. Alexopoulou, L., Holt, A. C., Medzhitov, R. & Flavell, R. A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413, 732–738 (2001).
22. Poltorak, A. et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 (1998). The first report that TLR4 is involved in the recognition of bacterial components.
23. Kawasaki, K. et al. Mouse Toll-like receptor 4–MD-2 complex mediates lipopolysaccharide-mimetic signal transduction by taxol. J. Biol. Chem.
275, 2251–2254 (2000).
24. Kurt-Jones, E. A. et al. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nature Immunol. 1, 398–401 (2000).
25. Rassa, J. C. et al. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl Acad. Sci. USA 99, 2281–2286 (2002).
26. Bulut, Y. et al. Chlamydial heat shock protein 60 activates macrophages and endothelial cells through Toll-like receptor 4 and MD2 in a MyD88-dependent pathway. J. Immunol. 168, 1435–1440 (2002).
27. Ohashi, K., Burkart, V., Flohe, S. & Kolb, H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164, 558–561 (2000).
28. Vabulas, R. M. et al. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 277, 15107–15112 (2002).
29. Okamura, Y. et al. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276, 10229–10233 (2001).
30. Termeer, C. et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J. Exp. Med. 195, 99–111 (2002).
31. Johnson, G. B., Brunn, G. J., Kodaira, Y. & Platt, J. L. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J. Immunol. 168, 5233–5239 (2002).
32. Smiley, S. T., King, J. A. & Hancock, W. W. Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J. Immunol. 167, 2887–2894 (2001).
33. Hayashi, F. et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor-5. Nature 410, 1099–1103 (2001).
34. Takeuchi, O. et al. Discrimination of bacterial lipopeptides by Toll-like receptor 6. Int. Immunol. 13, 933–940 (2001).
35. Schwadner, R. et al. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274, 17406–17409 (1999).
36. Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl Acad. Sci. USA 97, 13766–13771 (2000).
37. Hemmi, H. et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nature Immunol. 3, 196–200 (2002).
38. Heil, F. et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily.
Eur. J. Immunol. 33, 2987–2997 (2003).
39. Heil, F. et al. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 33, 2987–2997 (2003).
40. Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
41. Diebold, S. S., Kaisho, T., Hemmi, H., Akira, S. & Reis e Sousa, C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 (2004).
42. Jurk, M. et al. Human TLR or TLR8 independently confer responsiveness to the antiviral compound R-848. Nature Immunol. 3, 499 (2002).
43. Heil, F. et al. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 (2004).
44. Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
45. Zhang, D. et al. A Toll-like receptor that prevents infection by uropathogenic bacteria. Science 303, 1522–1526 (2004).
46. Science 303. (5663):1526-9.
47. Uematsu S., Akira S. (2008) Toll-Like Receptors (TLRs) and Their Ligands. In: Bauer S., Hartmann G. (eds) Toll-Like Receptors (TLRs) and Innate Immunity. Handbook of Experimental Pharmacology, vol 183. Springer, Berlin, Heidelberg.Manufacturing Practice, 2004