Sommaire
- The alternatives and rapid Microbiological methods & Pharmacopeias world
- Industry pivots to rFC adoption: Reshaping the Endotoxin Test & Regulatory Landscape
- Rapid Bioburden & Sterility Testing: exploring Detection Limits of an Automated Solid Phase Cytometry System
- The Myth of Testing Colored Samples: Debunked
- Design of smart galenic models (stimuli-sensitive) for the formulation of active molecules
- How process sufficiency can help pharma industries achieve their carbon footprint reduction targets?
- Advancing Sustainability: Recycling Single-Use Technology in the Pharmaceutical Industry
Industry pivots to rFC adoption: Reshaping the Endotoxin Test & Regulatory Landscape
The absence of compendial recognition by the USP has slowed down the widespread adoption of rFC, impacting not only domestic pharmaceutical companies but also posing challenges for European entities seeking to export products beyond the EU. However, the imminent embrace of rFC within the USP (Draft USP <86>) represents a transformative moment, aligning global industry initiatives with regulatory momentum.
The evolving regulatory landscape is witnessing a pivotal shift, particularly with the imminent inclusion of Recombinant Factor C (rFC in the United States Pharmacopeia as USP <86>). This change signifies a significant breakthrough as rFC gains compendial recognition, aligning with its existing status in the European Pharmacopoeia (EP). The convergence of these standards signifies a broader acknowledgment of rFC’s efficacy, indicating a potential for global harmonization in a near future(3).
The global pharmaceutical market has expanded substantially over the last two decades, growing sixfold from $113 billion in 2000 to $629 billion in 2019. With this growth, however, comes increased vulnerability in terms of supply chain security. As more companies rely on a complex network of global suppliers, the need for robust and resilient strategies becomes paramount. A recent survey by the McKinsey Global Institute highlighted concerns about sole sourcing and the lack of visibility into supplier risks, signifying critical vulnerabilities within the industry(1). The motivation behind the adoption of rFC is multifold. Industry leaders recognize the urgency to (a) diversify their supply chains(2) (b) fortify their resilience, addressing concerns related to geopolitical threats, (c) achieve environmental sustainability, and (d) assure global test performance uniformity. The limited availability of horseshoe crabs essential for Limulus amebocyte lysate (LAL) production poses a critical risk. With both climate challenges as well as geopolitical tensions potentially impacting the accessibility of these marine animal-derived products. The adoption of rFC emerges as a proactive measure to secure a consistent supply of testing reagents for pharmaceutical products(3,4,5).
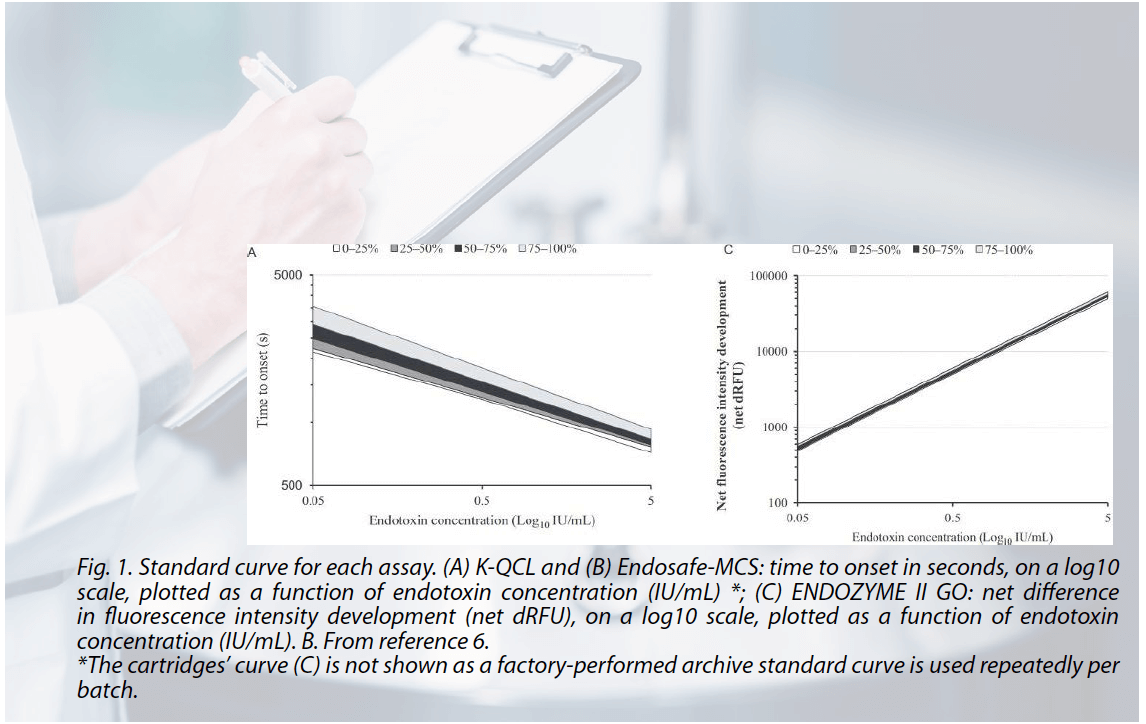
Moreover, the inherent consistency of rFC derived from biotechnological production stands in stark contrast to the variability associated with materials sourced from the wild. Natural source variability includes animal diversity (sex, weight, diet, etc.) as well as production-related extraction and formulation-associated variation. Recombinant reagents offer a level of consistency crucial for maintaining uniform test results, characterized by their stable performance across batches, as opposed to the inherent variations in naturally harvested materials. The rFC reagent has been demonstrated to be much less variable in terms of repeating constant standard curve properties related to batch after batch testing as shown in Fig. 1.
The urgency of adopting rFC is underscored by the escalating demand for LAL in the wake of the global surge in COVID-19 vaccine and treatment testing(7). Biomedical harvesting of horseshoe crabs has grown 27% full-year over year (now at 911,826) as per the ASMFC report on the 2022 Fishing year (Oct. 2023, Table 2, page 8). Efforts to expand horseshoe crab harvest areas to meet this demand are straining the ecosystem, threatening the delicate balance of shorebirds dependent on this keystone species(8,9,10). Recognizing the challenges in transitioning entirely from LAL, many companies opt for a phased approach to meet their sustainability and supply chain assurance goals. They integrate rFC for water, raw material, and container closure testing endotoxin testing while continuing to use LAL for legacy finished products., Water, raws, and container closures constitute a significant portion of endotoxin tests (>80%). The wide range of non- finished products that can be tested without regulatory permission (classified as “inspectional”) allows for a seamless transition without incurring substantial regulatory barriers(3). The imminent inclusion of rFC in the USP demonstrates the industry’s proactive perspective towards embracing sustainable solutions. As the pharmaceutical sector increasingly integrates rFC into its practices, the path toward global regulatory acceptance becomes clearer, serving to fortify supply chain robustness. The momentum toward adopting rFC represents a critical mass, where industry and regulatory bodies converge to prioritize resilient, sustainable, and consistent endotoxin testing methods. This joint effort marks a significant step forward in reshaping the landscape of pharmaceutical testing and highlights the imperative for adaptive, sustainable, and globally accepted methods(1,4,7).

References
- (1) Foster T, Patel P, Skiba K. Four ways pharma companies can make their supply chains more resilient. Pharmaceuticals & Medical Products Practice. McKinsey & Company. 2021.
- (2) Lakovou E, Chelsea C. White III, How to build more secure, resilient, next-gen U.S. supply chains. Brookings Tech Stream, December 3, 2020. https://www.brookings.edu/techstream/ how-to- build-more-secure-resilient-next-gen-u-s-supply-chains
- (3) Marius, Vacher, Bonnevay. Comparison of bacterial endotoxin testing methods in purified pharmaceutical water matrices. Biologicals, Volume 67, Sept. 2020, Pages 49-55.
- (4) Eisner C. Of McMaster and Marshes: Inside the $500K proposal to bleed protected SC horseshoe crabs. The State, Sept. 5, 2022. https://www.thestate.com/news/state/south- carolina/article257214377.html#storylink=cpy
- (5) Galvin WF. Horseshoe Crab Blood Extraction Facility Proposed. The Cape Cod Chronicle. April 6, 2022.
- (6) Galvin WF. Despite Mortality Concerns, Horseshoe Crab Blood Facility Gets Permit. The Cape Cod Chronicle. April 20, 2022.
- (7) Earls M. Pharma Lab Seeks Emergency Order to Begin Horseshoe Crab Harvest. Bloomberg Law. May 18, 2021. https://news.bloomberglaw.com/environment-and-energy/pharma- lab-seeks-emergency- order-to-begin-horseshoe-crab-harvest
- (8) “Revision to the Framework for Adaptive Management of Horseshoe Crab Harvest in the Delaware Bay Inclusive of Red Knot Conservation.” ASMFC, Adaptive Resource Management Subcommittee. Draft, 2021. Framework Revision. Page 28. http://www.asmfc.org/files/Meetings/2022WinterMeeting/HorseshoeCrabBoard_Jan2022.pdf.
- (9) Watson J, Tangley L. Shorebirds’ Fate Hinges on Horseshoe Crabs. Conservation (NWF). Sep 30, 2013.
- (10) Without enough horseshoe crabs, a threatened bird could go extinct. The State We are in (NJCF). August 25, 2022. https://www.njconservation.org/without-enough- horseshoe- crabs-a-threatened-bird-could-go-extinct/




