Summary
- ASTM E2500 : let's cross the Bridge !
- From study of the context to use of appropriate water treatment technologies
- A new water management strategy for the pharmaceutical industry
- Continuous measurement in water for pharmaceutical use (WPU)
- Candida auris, an emerging pathogenic “killer” species
- Cleaning and disinfection program part of the lifecycle approach: a risk based rather arbitrarily imposed approach
- Pharmaceutical water: the inside story of installation maintenance
- Pharma & the New Healthcare Economy. Ready or not ?
It will come as a surprise to nobody that water is a crucial issue for the pharmaceutical industry. Whether it is used for dry forms or liquid forms, as an excipient in non-sterile liquid products, in cleaning solutions or as a starting material for injectable medicinal products, it is omnipresent. It may be present in 80% of medicinal product formulations.
ccordingly, water should be the focus of particular attention when a manufacturer identifies a production requirement. The purpose of this article is to explain the methodology to be applied, from determination of your needs through to the set-up of a suitable and reliable water production system.
1. Regulatory constraints and opportunities
The pharmaceutical industry is very often governed by procedures and standards. Its water production systems are no exception to the rule. From “raw” water to the water used in its final application, regulations need to be followed, creating – depending on how they are perceived – either constraints or, alternatively, opportunities.
1.1 Types of water
“Raw” water – i.e. the water with which the manufacturer starts the process, must be potable. No criteria will be explicitly detailed here because liquid water is said to be potable when it presents certain characteristics (chloride concentration, pH, temperature, etc.) making it fit for human consumption.
The reference standards in this respect differ depending on periods, countries and, in some countries, the authority responsible for the definition1. The concept of “potability” varies around the world, as a result of the historic, scientific and local cultural context.
While no local authorities issue water standards, the World Health Organization criteria represent the gold standard.
Produced water, on the other hand, is governed by very clear texts: the pharmacopoeias and their monographs. The standards vary depending on the water’s application.
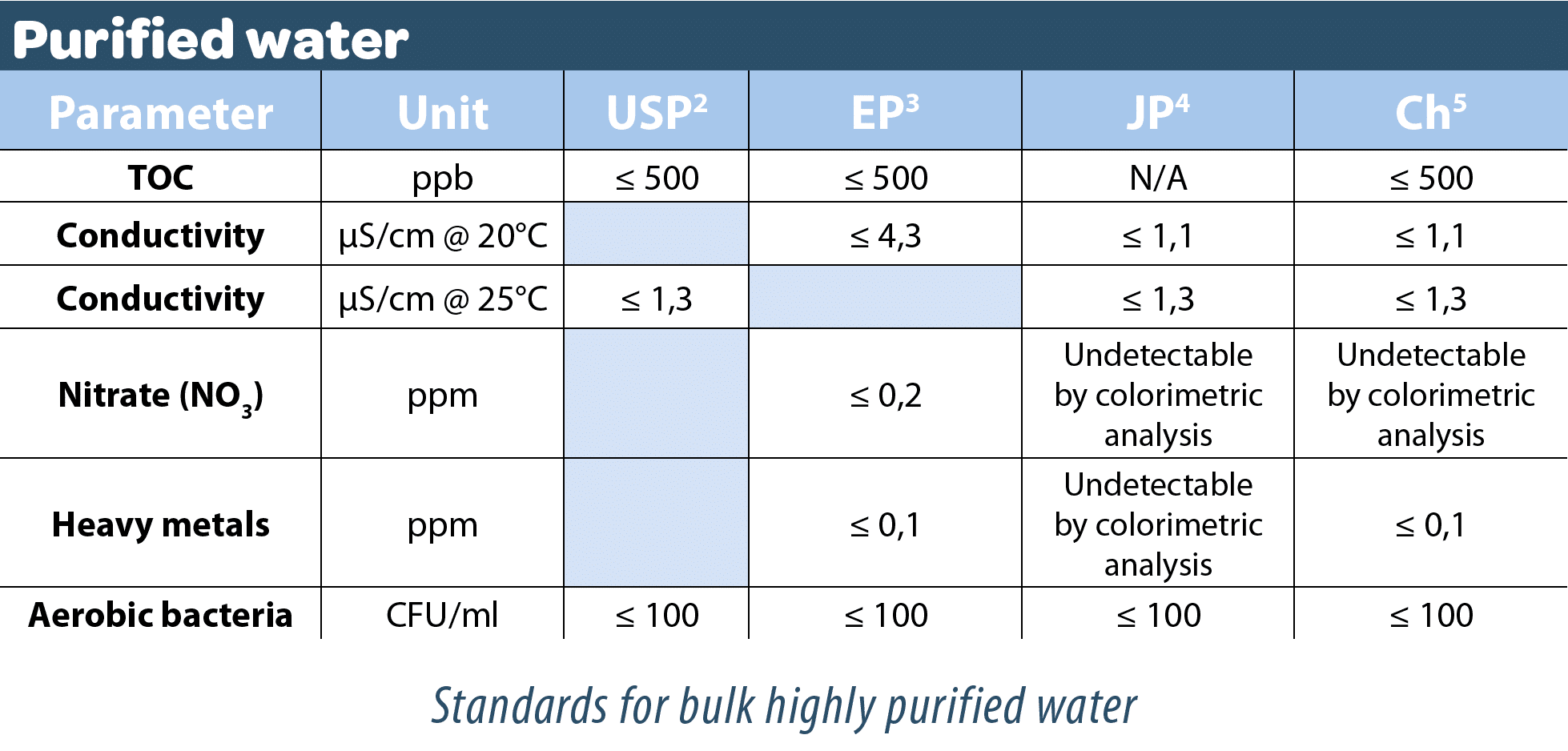
Bulk purified water
It may be produced by distillation, ion exchange or any other appropriate process from water complying with the regulations relative to water intended for human consumption. It does not contain additives. This bulk purified water is used for the manufacture of active substances and dry forms, as an excipient in non-sterile, non-apyrogenic liquid products and as a cleaning and rinsing solution for preparation equipment.
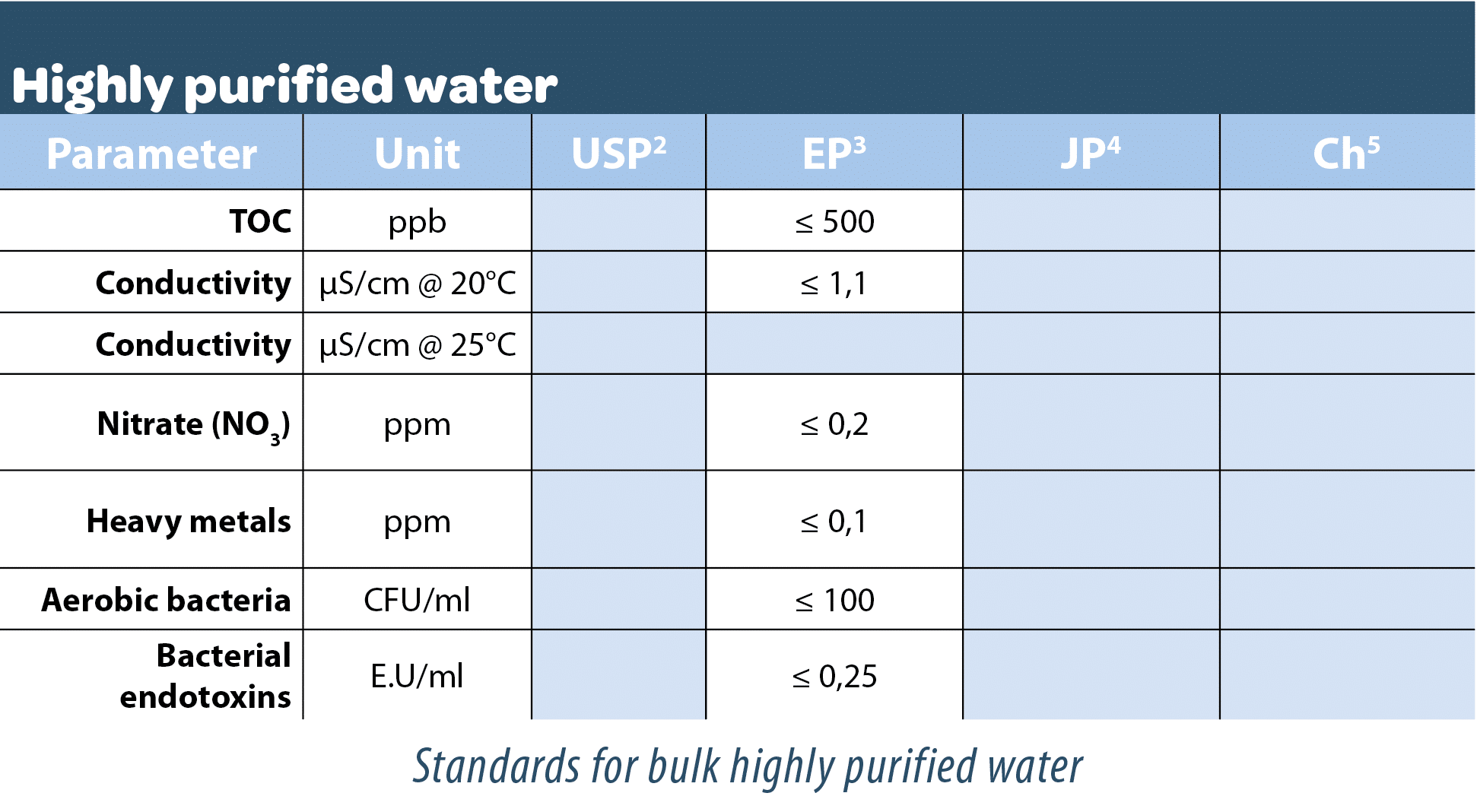
Bulk highly purified water
It may be produced by any suitable process (with ultrafiltration being one of the safest methods combined with others) from water complying with the regulations relative to water intended for human consumption. It does not contain additives.
This bulk highly purified water is used for the manufacture of active substances and dry forms, as an excipient in non-injectable, apyrogenic liquid products and as a cleaning and rinsing solution for preparation equipment. As a general rule, this water is used when a high biological quality is required. However, it is not used in the manufacture of sterile medicinal products unless specifically authorized by the competent authorities.
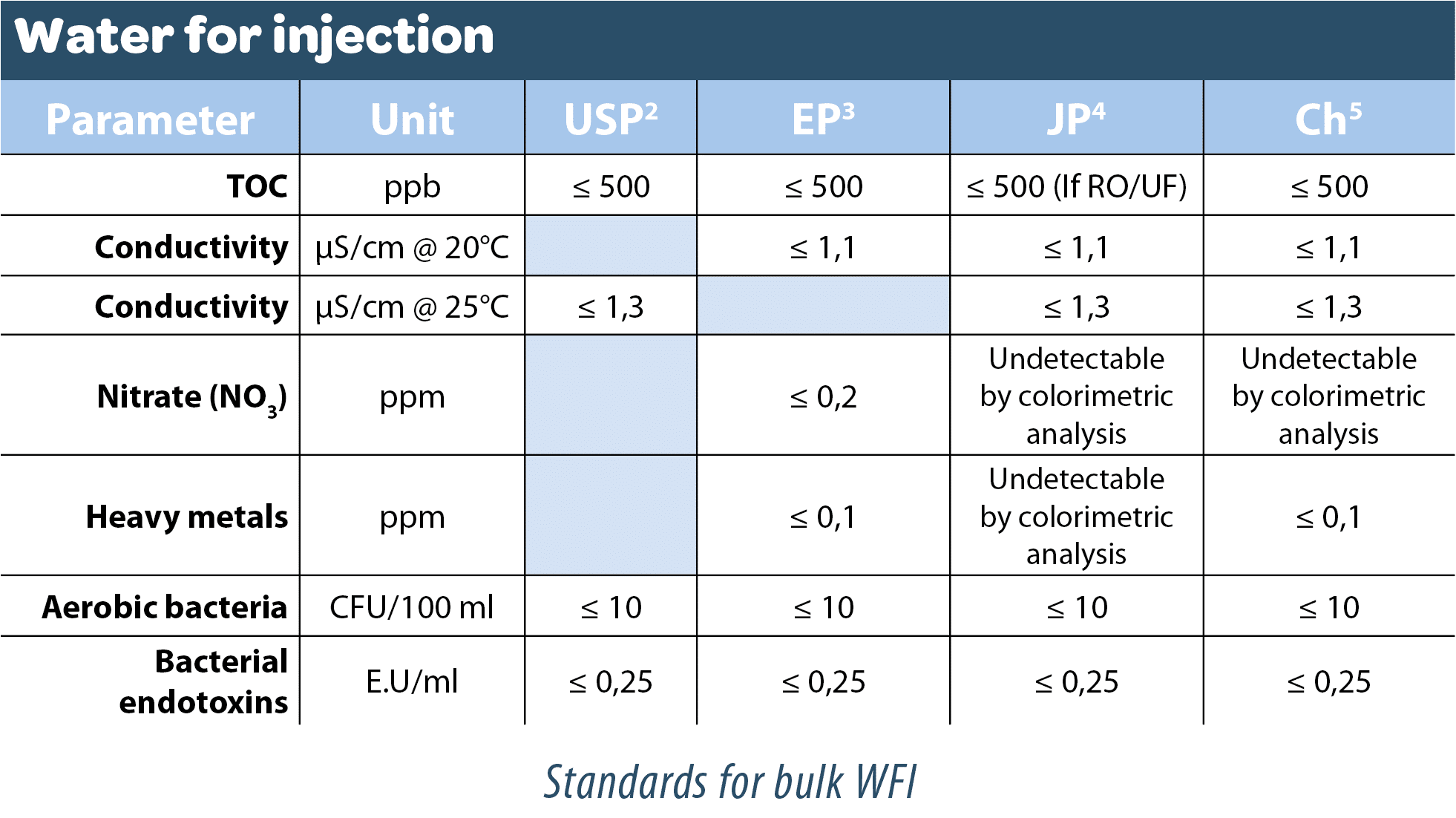
Bulk water for injection
Although for many years this differed from bulk highly purified water because it had to be imperatively prepared by distillation (European Pharmacopoeia), the European Pharmacopoeia commission of the Council of Europe ruled in March 2016 that this distillation process would no longer be mandatory from April 2017. This change brought it into line with the US Pharmacopoeia.
Thus, although the quality criteria have not changed, the authorized processes have evolved to include membrane processes coupled with electrodeionization and ultrafiltration steps.
1.2 Opportunities
Although, at first sight, obtaining a highly regulated physicochemical and bacteriological quality may appear to be complicated when starting with a potable water that is, by definition, variable for these same criteria, regulatory changes, such as the revision of the European Pharmacopoeia’s monograph for water for injection, propose a broad range of technological solutions, each with its own advantages and disadvantages. These concern investment costs, operating costs, reliability, TCO (Total Cost of Ownership), etc. Reflection is therefore required on this topic.
2. A clear strategy
2.1 Definitons
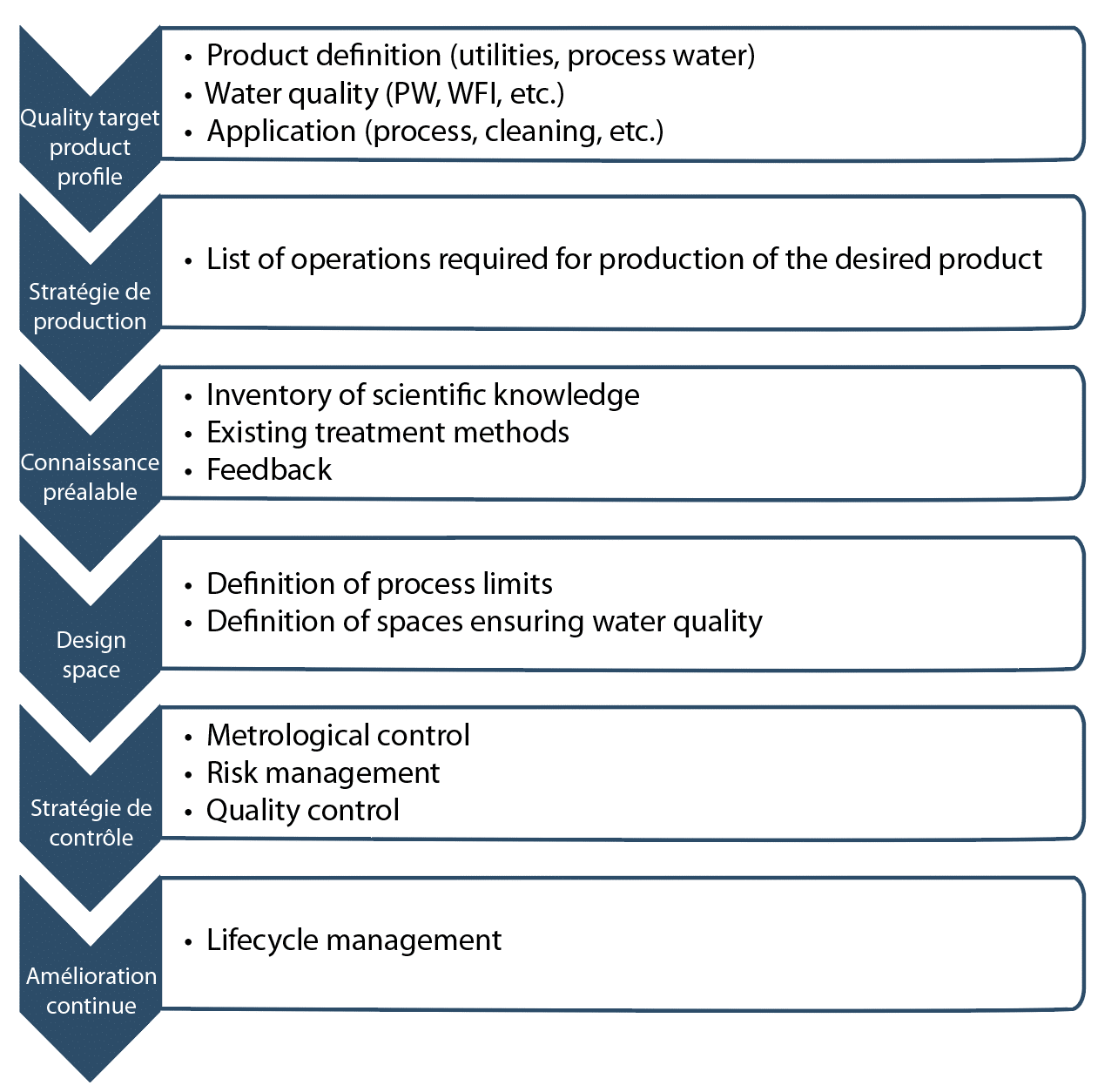
For any strategy, an action plan is required and the list of tasks to be accomplished can be summarized as in Figure 1. When this methodology is applied to water treatment systems, one rule always applies. We must start at the end (end users) and go back to the beginning (my potable (drinking) water quality and my production system).
2.2 Essential prerequisites and the art of summarizing
All the information specific to your case is essential to determine the optimum sequence and can be summed up in the form of a checklist:
- What medicinal product am I producing and which monograph is it associated with?
- What is my amortization timing?
- What utilities do I have access to and what are their costs (iced water, steam, potable water, etc.)?
- How much space do I have?
- What is the level of training of my teams in a given technology?
- Define a precise timeline of my water requirements (quantity, flow rate, time, number of users, temperature)
- What is the quality of my water supply?
On the latter point, it should be noted that a single analysis would appear to be highly inadequate in terms of aiding design definition. In fact, seasonal variations and networking between cities require a test campaign run over a full year, enabling assessment of the most restrictive values on water parameters such as its clogging power, its mineral content and its silica concentration, etc.
3. Technological choices
Once again, we will start at the end to go back to the beginning and it is necessary to incorporate all the criteria listed below to make effective decisions.
3.1 Choice factors
It should be noted that this comparison is based on direct costs only. The costs of external analyses are not taken into account here, …
Will my water be used hot or cold?
Although purified water is generally distributed cold and water for injection hot, it is important to know who uses what quantity of water and at what temperature(s) in order to best determine the temperature maintenance regime and, consequently, the correct maintenance of microbial load via disinfection methods.
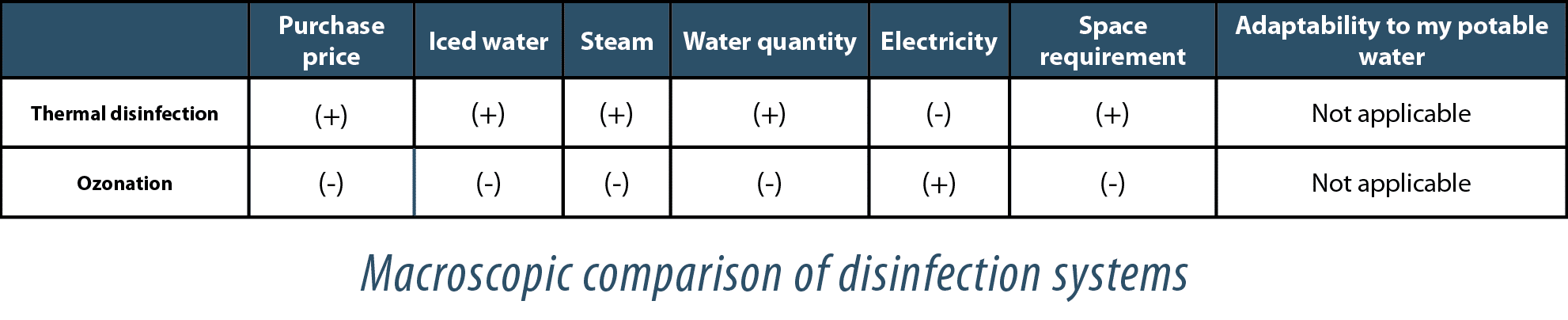
Disinfection systems available
Although a simple interpretation of the table would tend to naturally orient the decision towards ozonation, this needs to be weighed up against the water’s use temperature, the time available for this operation and the cost of your utilities.
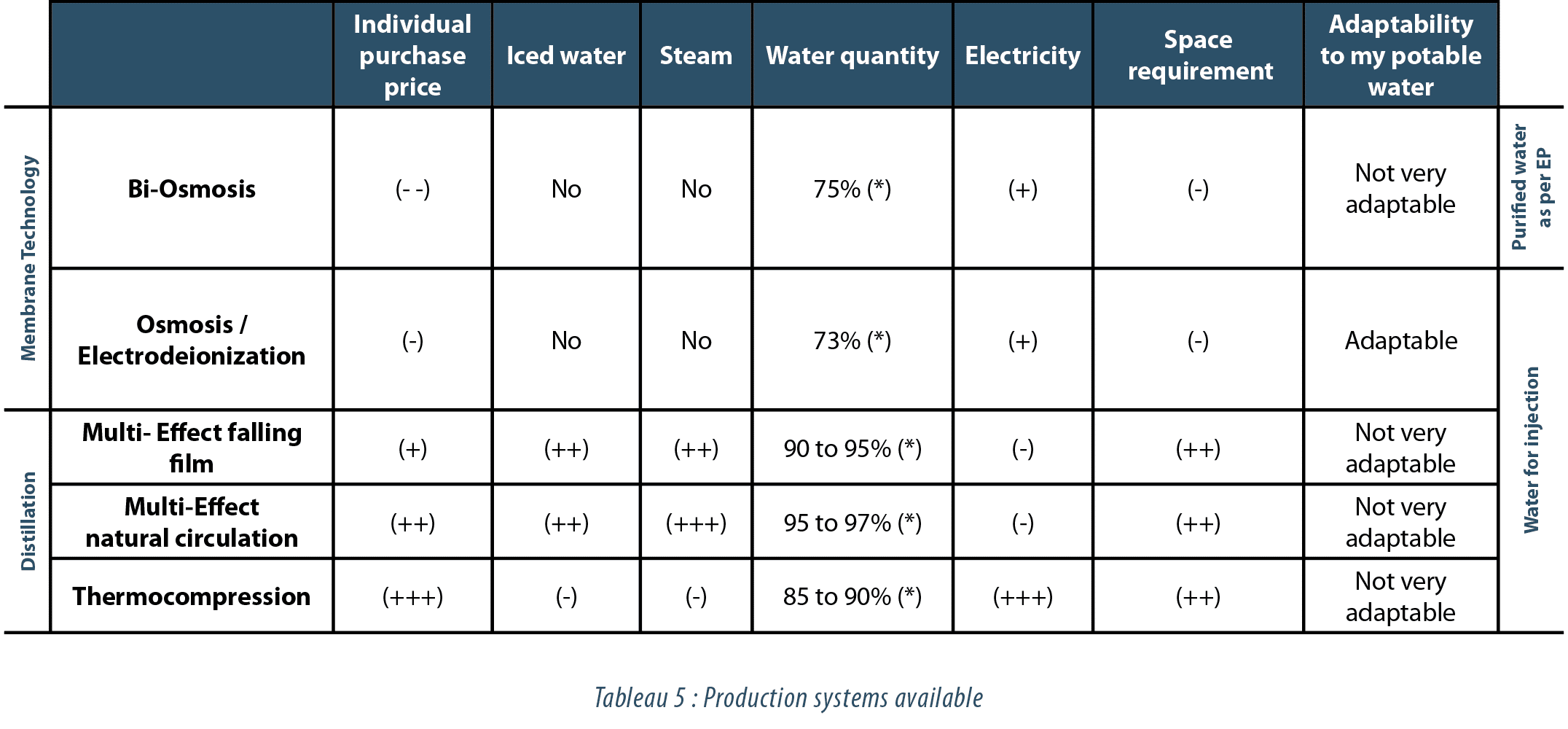
Production systems available
(cf. tableau 5)
3.2 For bulk puried water
It is accepted that for the production of purified water in bulk, the following technologies can be considered:
- Bi-osmosis
- Osmosis coupled with electrodeionization
- Thermocompression
In view of the comparison below (see table 5), it very clearly appears that the TCO/ CAPEX ratio can be a powerful decision-making criterion if producers are taken individually. Utility consumption, available space and the quality of your potable water are also key factors. High mineral contents, the presence of silica and a marked variability are all criteria that naturally guide your choices.
3.3 For water for injection
“Water for injections […] is obtained from water that complies with the regulations on water intended for human consumption […] or from purified water. It is produced either by distillation […] or a purification process that is equivalent to distillation. Reverse osmosis, which may be single-pass or double-pass, coupled with other appropriate techniques such as electrodeionization, ultrafiltration or nanofiltration, is suitable. Notice is given to the supervisory authority of the manufacturer before implementation. For all methods of production, correct operation monitoring and maintenance of the system are essential. In order to ensure the appropriate quality of the water, validated procedures, in-process monitoring of the electrical conductivity, and regular monitoring of total organic carbon and microbial contamination are applied. The first portion of water obtained when the system begins to function is discarded.“
Source: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/08/WC500211657
Since April 2017, the European Pharmacopoeia has authorized technologies other than distillation. This is therefore a great opportunity to broaden the scope of options and the technologies that may be envisaged for the production of water for injection are therefore:
- Single-pass or double-pass osmosis, coupled with other electrodeionization combined with ultrafiltration
- The 3 types of distillation systems
While the issue of differences in produced water quality needs to be excluded (with each system capable of supplying an equivalent quantity), it is much more interesting to focus initially on the interest that “cold” production may represent. This will then lead quite quickly to the comparison above (see table 5), expressing cost/consumption differences…
A check list is also required to measure the interest represented by cold production:
- Sustainable development:to what extent is resource consumption important to you?
- Do your users use hot or cold water?
- Utilities costs: what is the proportion of utilities costs in total product cost?
- Regulatory authorization and requirements: how will I declare this to the authorities, and with what resources?
If your users primarily use hot water, if your utilities costs are marginal and if your amortization allows the purchase of a more expensive system, distillation systems should be favored since they are tried and tested in your plants, your technicians are better trained in them and this will not lead to any qualification differences. Conversely, if cold production is shown to be relevant, the following figures should be borne in mind when performing an internal study.
Study conditions:
- Amortization: 10 years; 300 days of operation/year; 20 hours of operation/day; Wastewater 2 €/m3; Energy: 0,2 €/kwh ; Industrial steam: 8 barg 175C: 35 €/t ; Iced water : 3 €/m³ ; Water supply: potable water (RO-RO-EDI) 1.50 €/m3, Purified water (Distillation) 5.70 €/m3
- For the production of 3.6 m3/h : the total saving made by switching from a distillation system to a membrane solution is in the region of -65% €/m3 of WFI.
- For a production of 103/h : the total saving made by switching from a distillation system to a membrane solution is in the region of -54% €/m3 of WFI.
The choice between these four technologies therefore needs to be carefully weighed up against the information collected at the start of the project and detailed in paragraph 2.2.
Conclusion
Over these few pages, we have seen to what extent a context study, specific to each case, affects all the decisions that will be made in order to obtain the most appropriate solution. This reveals the magnitude of the task and the serious need to characterize data that may appear to be simple but are actually relatively complex to compile, such as the real cost of your water and your utilities.

Pierre CULLMANN – BWT
A chemical engineer specializing in water treatment, Pierre’s main responsibilities are to support manufacturers in determining their needs in the production of water for pharmaceutical use, but also acts as a trainer and expert on design issues, qualification and technical assistance. Its field of action goes from France, to the Maghreb countries and on the follow-up of certain accounts all over the world for BWT.
pierre.cullmann@bwt.fr
Share the article
Sources
– EMA QA on production of water for injections by non-distillation method.._Ph-Eur 0169_draft_EN
– Source : www.ema. uropa.eu/docs/en_GB/ document_library/Scientific_guideline/201 /08/ WC500211657.pdf
Bibliography
[1]For example, in the USA, the FDA, the EPA or individual States do not have the same criteria and recommendations for potable water or for tap or bottled water.
[2] United states pharmacopoeia
[3] European pharmacopoeia
[4] Japanese pharmacopoeia
[5] Chineese pharmacopoeia