Sommaire
- Quelques bonnes pratiques de validation des procédures analytiques
- ALCOA… avec un A pour Accuracy
- Established Conditions for Analytical Procedures and QbD: current situation and future perspectives for enhanced change management paradigm
- Quand les vibrations des molécules permettent de les doser : la spectroscopie proche infrarouge en action
- Apport des études de stabilités accélérées prédictives (APS) au développement pharmaceutique
- Mise en place de la chimie analytique verte au laboratoire contrôle qualité de la société UPSA
- Évaporation des solutions à base d’alcool : Quels résidus sur les équipements ?
Established Conditions for Analytical Procedures and QbD: current situation and future perspectives for enhanced change management paradigm
Established conditions (EC) are defined for manufacturing and control as elements considered necessary to assure product quality (for EC related to manufacturing process parameters) and to assure method performance (for EC related to analytical procedure variables). ECs for analytical methods maintain reliable results that ensure the effcient product control strategy during the product life cycle.
Therefore, changing ECs of analytical methods require a certain level of regulatory activity as discussed later. This level of regulatory activity can be dramatically different depending on the type of changes, the ECs filed, as well as the strategy followed to build these ECs.
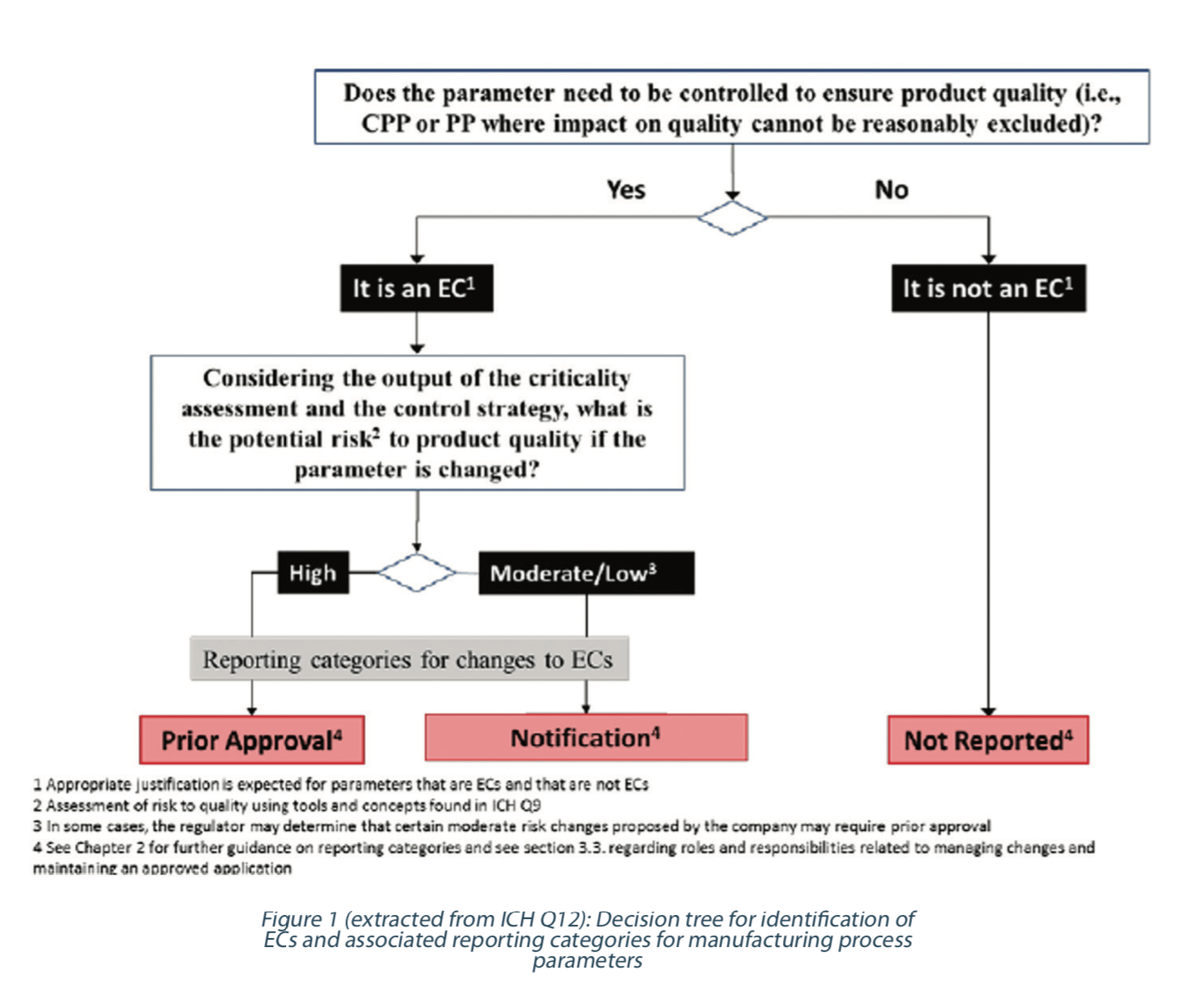
A harmonized scientific risk-based approach can be applied to define ECs for manufacturing processes and analytical procedures and their associated reporting categories. A decision tree which illustrates the specific approach to identifying ECs and reporting categories for manufacturing processes has been proposed by ICH Q12 (See Figure 1). In this paper, we will discuss how to translate this methodology to ECs for analytical methods. Then, we will share our view about what could be the evolution of EC and change management for analytical procedures in the future; when the use of ECs and analytical QbD principles will have reached a level of maturity allowing further improvements.
1. Application for analytical ECs
The principles described in this decision tree for the manufacturing process can be applied to identify ECs for analytical methods. The extent of ECs and their reporting categories could vary depending on the degree of the understanding of the relationship between method variables and method performance, the method complexity, and analytical control strategy. A justification to support the identification of ECs and corresponding reporting categories for changes to ECs and those that are not ECs based on risk management should be provided.
The recommended approach to identify ECs for analytical procedures is to apply the enhanced approach of analytical quality by design (AQbD) for method development. Based on development studies, risk analysis and robustness studies, the relationship between the analytical procedure variables (for example analytical procedure parameters or operating conditions) and the performance of the analytical procedure can be demonstrated and completed by data generated during the life cycle of the analytical procedure.
Depending the relevance of the method in the product control strategy and on the risk represented by the method for product quality monitoring, an MODR can be established. Method operable design region (MODR) can be established for operating variables having numerical target (for example, incubation time and temperature, reagent concentration, centrifugation time or speed). Non-quantifiable variables may also be considered and evaluated in the risk assessment (for example references to or batches numbers of chromatographic columns, or of critical reagents). Results evaluation from risk analysis allows differentiation between critical and non-critical analytical procedure variables. This will allow the design of the method to be set, to define ECs and the associated analytical procedure control strategy. It is obviously very important to describe in ECs the critical variables affecting method performance as well as their associated MODR if appropriate (in case of numerical target). It is equally important for the non-critical variables to take the strategic decision between the two following options:
• Consider these variables as non-ECs or
• Consider these variables as part of ECs but being under control with a lower notification category.
These decisions are critical in order to obtain an alignment with authorities on the change level that will need to be considered when preparing a change affecting that method variable. ECs and non- ECs should be described in the dossier in the section related to the analytical procedure description and for non-ECs, justifications should be as solid as those provided for ECs.
During the life cycle management of the method, stage 1 (procedure design and development) and stage 2 (procedure performance qualification) are used to generate data, rationale, and knowledge of the method. Rationale and knowledge can be generic or specific in case they are related to the analytical technology or the matrix of the product. Data are always specific to a situation as they are linked to the analytical technology and the matrix of the product. The risk assessment and categorization applied at end of stage 2 allow identification of high and low risk elements. Control of changes of ECs and non-ECs will be managed during the stage 3 as part of the continued procedure performance verification (USP <1220>).
The proposed change management approach for a product as described in ICH Q12 can be applied to analytical methods. As for product, type of notifications to health authorities will depend to the categorization of the analytical method variables. These categorizations and the change strategy should be included in the dossier. For a non-EC, a change will not be reportable to the health authorities. Therefore, such change will need low effort and provide high benefits if recognized as low risk in the context of change. In case of an EC, if the change is related to a variable with a high risk on the method performance, submission will be needed and if the change is related to a variable with a medium or low risk, a notification will be acceptable. Additionally, for EC with a high-risk level, a refined strategy to reduce the level of change submission can be proposed. If during ECs registration, a post-approval change management protocol (PACMP) for change management is also submitted, that allows to reduce the high risk related to the change to a moderate or low risk of this change. As an example, it could be a change of reference standard or a change of chromatographic column, both could be controlled by a qualification protocol including a study design and acceptance criteria. This change strategy is presented in Figure 2.
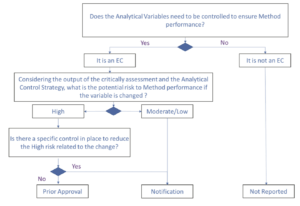
2. Changes affecting ECs
In this section we discuss the situation where EC are changed and the considerations around such changes. The principles of QbD lead us to consider the EC related to product attributes and the EC related to analytical procedures as intimately connected and thus the impact of a change in EC at the product level must be evaluated on the EC at the procedures level and reciprocally.
A change in EC related to a product attribute will obviously induce to challenge the fitness-for-purpose of the analytical methods measuring this attribute. The interconnection between QbD and AQbD workflows are illustrated in Figure 3.
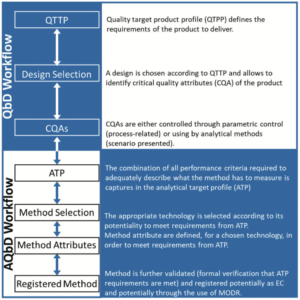
For instance the change in purification technology may change the impurities present in the product and that will lead to the revision of the ATP of existing procedures related to purity/impurity measurements or to the creation of a new ATP for an analytical procedure aiming to measure the new impurities. Moreover, this change in product matrix composition also leads to revise the ATP of all analytical procedures applied on this product production stage. After ATP revision, all analytical procedures will be assessed for capability to meet all ATP requirements in the newly described matrix conditions. This assessment will rely on 1) pre-existing generic information relative to the technology used for a given method, 2) pre-existing theoretical information relative to the specific procedure in use such as risk assessments, 3) existing experimental data on the specific procedure in use or 4) specifically generated experimental data on the specific procedure in use.
As illustrated above, this change in EC of the product might have multiples impact on analytical procedures It will also generate at least a peak of assessment and justification activities if not massive experimental analytical work. It is therefore reasonable to consider this change as an opportunity to revise a significant part of the control strategy in place and evaluate for instance, if selecting a new technology that may be used develop multi-attribute procedures. Indeed, this may finally present a case of positive return on investment and, on the long term, represent a more versatile technology regarding future similar changes in EC.
What about the reciprocal situation? Could the change of EC in analytical procedure induce a change of EC at the product level and therefore require a formal assessment? Even if less obvious and less frequent the answer can be yes. Two illustrative examples are presented hereunder.
When, in the context of the improvement of an analytical method, a change of technology or a change of nature of reference standard (e.g.: change from in vivo to in vitro potency in Vaccines) induces a recalculation of product specification expressed in the new referential used, the EC on the product must be adapted to reflect that change even if as such the product quality is not modified.
The second example is also in the context of a change of analytical technology that leads to discover yet unknown product properties. For instance, when thanks to the better resolution provided by a new type of HPLC column or by the translation of a method from HPLC to UPLC, a new chromatographic peak is resolved. This new peak will be characterized and might be identified as a new impurity, that may require testing in routine, and therefore included in product specification. This situation would require adapting ECs even if product quality remains unchanged.
Finally, as stage 3 allows to cumulate data and knowledge about the product and about analytical procedures, investing in a change in EC (at the product or the analytical level) should always be considered as an opportunity to revise ECs globally. Indeed, the new knowledge gathered over time might allow justified simplification of control strategy.
3. Perspective of AQbD on analytical EC
In this section we share our thoughts about what the evolution of EC and change management for analytical procedures in the future could be; when the use of EC and AQbD principles will have reached a level of maturity allowing further improvements. These visions are purely speculative but worth to be shared in order to stimulate reflection and discussion. It may also be used to stimulate the industry to engage into the application of current enhanced approaches as these first steps are essentials for allowing further evolutions of the systems ensuring the quality of the pharmaceutical products, the efficacy of the pharmaceutical supply chains and the efficient dialog between industries and health authorities.
Digitalization. One significant element coming into the global picture is data digitalization. In this discussion we will focus on analytical data but most of the elements can easily be translated to other types of data from the pharmaceutical industry. Increasingly industries are digitalizing their data allowing to massively cumulate, structure, organize and – as a more impactful consequence – analyze information that was present but hibernating into well-arranged paper binders waiting for the next inspection or investigation to reveal its tremendous contributing value to the understanding of the effectivity of a quality system and all activities related.
When all these data will be digitally available, it will be possible to perform massive retrospective data analysis exercises which – if properly designed – could transform these data into knowledge to be used for improving analytical procedure performances and also the control of these analytical procedures through the identification of yet unknown or underestimated analytical control attributes. Moreover, when these data will be instantly available digitally, the current practices used for analytical control strategy (e.g. control charting, system suitability) could/should be adapted according to the new possibilities provided by instant access to a comprehensive set of analytical control attributes. The direction of these adaptation could/should be the early or the instant detection of changes in procedure performance or even the prediction of changes in procedure performances based on multivariate analysis of a complete set of analytical control attributes. Allowing to move from a paradigm where method performances are measured at the moment of procedure validation (and then deemed as being maintained through surrogates) to a new paradigm where methods performances are evaluated in a continuous manner through digital exploitation of routine data and specific analysis of analytical control attributes.
Analytical Quality by Design is the other transformative element that is being put in place. As presented in USP <1220> and in several stimuli articles from USP, the principles of AQbD allow to develop better analytical procedures through systematic use of quality risk management together with oriented knowledge production that allows to control/lower the risks identified. Another important outcome expected from the AQbD approach is the knowledge generated and the identification/objectivation of the risks per se. These are critical elements that are generated during stage 1 (analytical procedure development) and stage 2 (analytical procedure qualification/ validation) and that will be used and maintained/enriched during the entire remaining life of the procedure: stage 3.
AQbD principles insist on the knowledge-driven development and implementation of an Analytical Control Strategy to ensure the procedure performs as expected and also on the effectiveness of ACS – together with risk assessment and knowledge available – and its role in determining the level of activities required to confirm that a changed analytical procedure is producing fir-for-purpose data.
Considering these AQbD principles and the potentialities offered by digitalization described above, one can imagine that ACS in use may be part of EC and that submission will be of a different level if normal or enhanced ACS is applied. Indeed, currently the risk related to a change is mitigated / objectivated through a qualification or validation study that is performed “offline” from routine activities and that is, in fact, a prediction of the impact of the implementation of the change. The decision to implement is taken based on data predicting that implementing the change will not affect significantly the performances of procedure. In case of failure in the prediction study, it is only after implementation that this failure will be detected. If enhanced ACS are implemented on one hand, and if knowledge is increased to a level allowing better risk assessment on the other hand, more changes should be eligible as requiring less preliminary qualification or validation activities and lower submission levels.
The evolution of safety elements in the automotive is a good illustrative example of this purpose. In a recent past, safety of a car was relying mainly on the management of impact of accidents: a crash test measured the level of protection of the passengers in case of crash. And the driver had to be skilled and fortunate enough to reduce risk of occurrence. Today, improvements are still made on the management of the impact but a lot is happening to reduce the occurrence of the risk trough systems helping the driver, compensating for his potential inattentions, mistakes or sleepiness and even ultimately compensating for his lack of skills by driving safely the car without his intervention. Is the pharmaceutical world ready to move from the crash test era betting on robust validated procedures to an era where validated robust procedures will be controlled using systems measuring continuously procedure performances allowing therefore seamless changes throughout lifecycle? Today, we are certainly not yet ready, but we need to take the first step now and adopt the current proposals for enhanced approaches and start the work and the dialog leading to the next era.
Conclusion
The principles of ECs and change management applied to analytical methods will allow regulators and companies to know exactly what will be provided or not and what is expected for a given pre-defined change. The implementation of EC for a product / method represents a real benefit for the patients, the health authorities and the pharmaceutical manufacturers and should be considered as a cornerstone for analytical life cycle management.
Disclosure
This work was sponsored by GlaxoSmithKline Biologicals SA. Jean-Francois Dierick is an employee of the GSK group of companies.
Partager l’article

Jean-François Dierick – GSK Vaccines
Jean-Francois Dierick is Global Subject Matter Expert for Analytical Validation & Lifecycle at GSK Vaccines. He is owning GSK Vaccines processes related to analytical method validation and method lifecycle (assay transfer, change management, performance monitoring…) and this across 10 commercial and 3 development sites. He is participating in the deployment of Analytical QbD and is leading/supporting several programs aiming at developing digital tools to enhance the monitoring of analytical methods and support/secure change management.
jean-francois.m.dierick@gsk.com

Isabelle Moineau – Aktehom Sante
Isabelle Moineau is working as an Analytical Expert Consultant for AKTEHOM. She has a PhD in Biochemistry from the University of Lyon specialising in analytical areas. She has been successfully working for over 15 years in Pharmaceutical industry and has gained a vast, solid professional experience. As analytical leader at AKTEHOM, Isabelle is leading the Analytical Quality by Design implementation in pharmaceutical industries in Quality Control and Development laboratories. She is a successful project Leader and she stimulates any mission team with a positive, innovative approach that results in a productive, efficient team effort to respond to the clients exact needs. She listens, guides and assists customers to evolve with the ever changing Pharmaceutical industry.
isabelle.moineau@aktehom.com
Acronyms
EC: Established Conditions
ATP: Analytical Target Profile
QbD: Quality by Design
AQbD: Analytical Quality by Design
CPP: Critical Process Parameter
PP: Process Parameter
MODR: Method Operable Design Region
ACS: Analytical Control Strategy
References
Concept paper ICH Q14: Analytical procedure development: Text & methodology Proposed new USP General Chapter
<1220>: The Analytical Procedure Lifecycle Pharmacopeial Forum 42(5): Stimuli to the Revision Process: Analytical Target Profile: Structure and Application Throughout the Analytical Lifecycle
Forum 42(5) Stimuli to the Revision Process: Analytical Control Strategy
ICH Q12: Technical and regulatory Pharmacopeial considerations for pharmaceutical product lifecycle management
Concept paper ICH Q2(R2): Validation of analytical procedures: Text & methodology Patrick Jackson et al. 2019, Analytical Chemistry 91 pp 2577-2585


