Sommaire
- Biotherapy. Médicaments de Thérapie Innovante et Annexe 1 : Analyse de son applicabilité
- How Can the Industry Drive Down the Cost of Goods to Better Serve the Patients?
- Maintaining contamination control in advanced therapy medicinal product manufacturing
- A Plug-and-Produce GMP Plant for Cell and Gene Therapy
- Maîtrise des risques : Stratégies et innovations pour sécuriser le développement des biomédicaments
- Statistical Approach to Aseptic Process Simulation: Representativeness and Proactive Alert Limit Setting for Aseptic Interventions
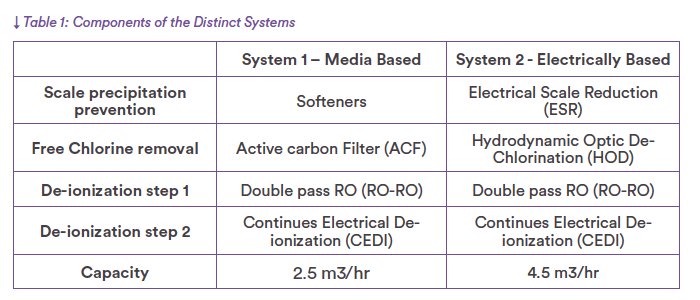
- Comparative Study of WFI Pretreatment Performance Electrically Based Pretreatment Outperforms Media-Based Pretreatment
- Détection à 100% des défauts critiques PP
- L’industrie pharma doit réduire sa trace carbone, ... le traitement d’air. Part 1
Comparative Study of WFI Pretreatment Performance Electrically Based Pretreatment Outperforms Media-Based Pretreatment
The generation of Purified Water (PW) and Water for Injection (WFI) is of significant importance in the pharmaceutical industry, as they are essential components used in various processes within pharmaceutical plants. It is crucial to implement appropriate methods and utilize equipment specifically designed to ensure that the generation process of PW remains free from microbial contamination. As the specification of the WFI microbial levels are lower by 3 orders of magnitude than the PW levels, the criticality of a clean operating WFI pretreatment system is far higher. In this article, the performance of the WFI generation will be compared.
The chemical criteria to be met are conductivity below 1.3µS/cm@25°C and TOC below 500 ppb(1). The microbiological criteria are maximum total count below 100 cfu/100ml with absence of pathogens(2) and Endotoxin below 0.25 EU/ml.

In this comparative study, we examine two distinct water systems designed to generate WFI. Both systems employ a double pass Reverse Osmosis (RO-RO) followed by Continuous Electrical Deionization (CEDI) as the final purification stage.
However, they differ in their RO pretreatment.
System 1 incorporates Softeners to remove calcium and magnesium to prevent scale precipitation in the RO. An Active Carbon Filter (ACF) follows the Softener to remove free chlorine from the municipal feed and so protects the membranes from oxidization.
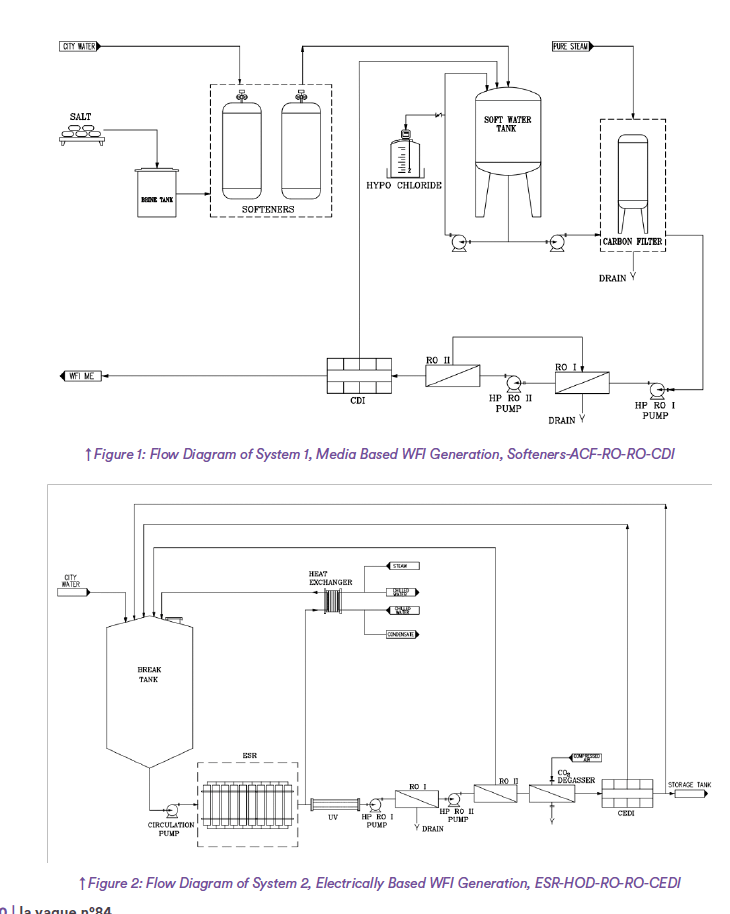
System 2 relies on Electrolytical Scale Reduction (ESR) to mitigate scale formation in the RO, supplemented by Hydro dynamic Optic De-Chlorination (HOD) to remove free chlorine. The ESR splits water and the ensuing pH polarity in the reactor precipitates the scale before the RO. The HOD iridates the water with a very high dosage of UV that destroys the free chlorine and renders it safe before it enters the sensitive RO membranes.
System 1 is constructed using stainless steel piping with chemically sanitizable membranes. Only the CEDI unit can be hot water sanitized to ensure control of the final microbial levels.
System 2 is constructed using pharmaceutical-grade stainless steel units, ensuring compliance with industry standards. This includes hot water sanitizable pretreatment (ESR-HOD) and production system (RO-RO-CEDI) units to guarantee effective purification and sanitation.
Additionally, as both systems are installed in the same plant, they both utilize identical municipal feed water, facilitating a direct comparison between the two approaches.
The equipment combinations for each system are detailed in Table 1.
As the RO-RO-CEDI s are similar in both systems the pretreatment equipment is the main deciding factor in the different performances.
Through this comparative study, our objective is to reinforces the assumption that the performance of systems with media-based pretreatment is inferior to Electrically Based pretreatment.
We will conduct a comparison of micro biological parameters and evaluate the performance, efficiency, and suitability of the pretreatment stage in the two water systems.
The findings from this study will contribute to the advancement of water pretreatment technology and support the generation of PW and WFI in the healthcare industry.
1. Systems Descriptions
In System 1, with the softener and ACF pretreatment, the feed bacteria is removed by the first stage RO. On the other hand, in System 2, the bacteria is first reduced by free chlorine generation in the ESR and then greatly reduced by the very powerful UV irradiation going through the HOD that is a very powerful UV. This ensures in system 2 that the membranes remain free from bacterial contamination and biological fouling.
In System 1, the CEDI equipment is hot water sanitized monthly, heating up to a minimum of 80°C and keeping the system hot for one hour before cooling
down.
The RO-RO is sanitized twice yearly basis with a 2% NaOH chemical wash.
The ACF is thermal sanitized weekly at 130°C for two hours.
In System 2, the total system including ESR, HOD and the RO-RO-CEDI equipment was automatically hot water sanitized on a weekly basis, heating up to a minimum of 80°C and keeping the system hot for one hour before cooling down.
The components technologies/equipment used:
System 1 Pretreatment:
The softeners are used to reduce the hardness of the water and to minimize the potential for scale forming on the surface of an RO membrane. Typically replace calcium (Ca2+) and magnesium (Mg2+) ions, which are the main contributors to water hardness, with sodium (Na+) ions. resulting in softened water with
reduced hardness, but the total load of ions on the RO is unchanged.
The Softeners are usually installed after the city water tank.
The Active Carbon Filter (ACF) is installed after the softeners and utilizes activated carbon to remove free chlorine from the water, ensuring chlorine-free feed water to the RO and CEDI. This prevents potential degradation of the RO membrane, as chlorine can have dama ging effects on its integrity and performance.
System 2 Pretreatment:
The Electrical Scale Reduction (ESR) technology effectively reduces scale in the RO feed water, minimizing the potential for scale precipitation in the RO membrane concentrate. It utilizes Stainless Steel (SS) reactors equipped with central electrodes. An electrical current is applied from the central electrode through the water to the cylindrical wall of the reactor, resulting in the dissociation of some water molecules into OH- and H+ ions4. Scale formation occurs on the reactor wall in regions with high concentrations of OH- (hydroxyl ions), which are associated with elevated pH levels. Some of the scale adheres to the reactor wall, while the rest settles at the bottom of the unit.
Hydro Optical Dechlorination (HOD)
The hypochlorous form of free chlorine is reduced through the following reaction using UV light:
2HOCl → 2HCl + O2 4,5
The installed unit employs medium pressure UV lamps that emit UV wavelengths primarily at 240 nm and 290 nm. These specific wavelengths correspond to local
maximums for the absorption of HOCl.
2. Comparison of microbial performance between System 1 and System 2
Comparison of Pretreatment
As System 1 has media pretreatment systems, System 1 has no protection against bacterial colonization in the RO. On the other hand, System 2 has low levels of free chlorine in the feed tank caused by the ESR activating some of the naturally occurring chlorides in the feed water according to the following formula:
Cl- + H+ → HCL , HCl + HCl → Cl2 + H23
This free chlorine by-product disinfects the ESR and destroys biofilm.
In addition, System 2 has a very powerful UV lamp, minimum dose of 1,400,000 µJ/cm2 that also disinfects and reduces
incoming bacteria before encountering the RO.
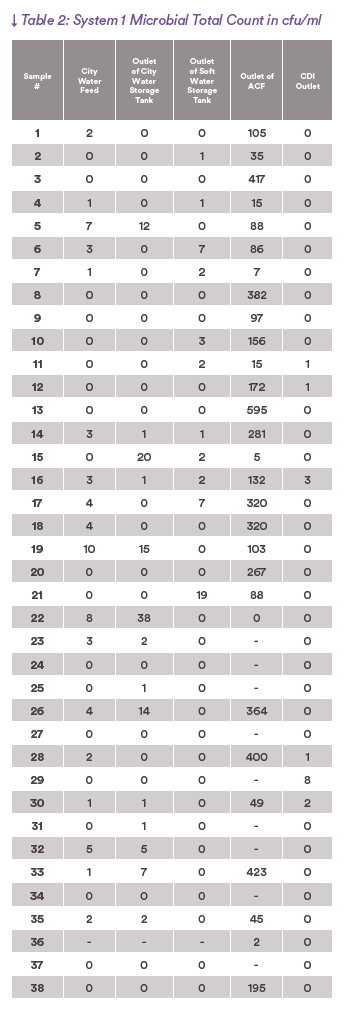
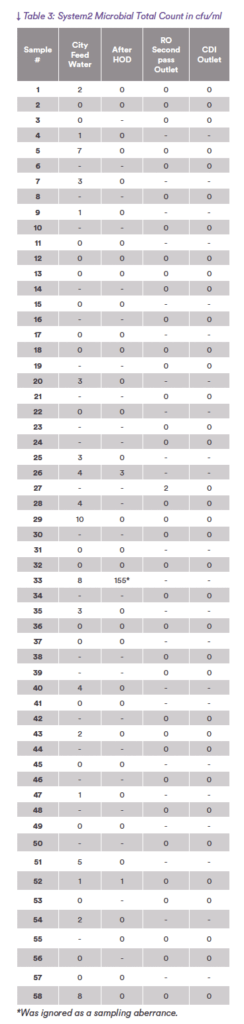
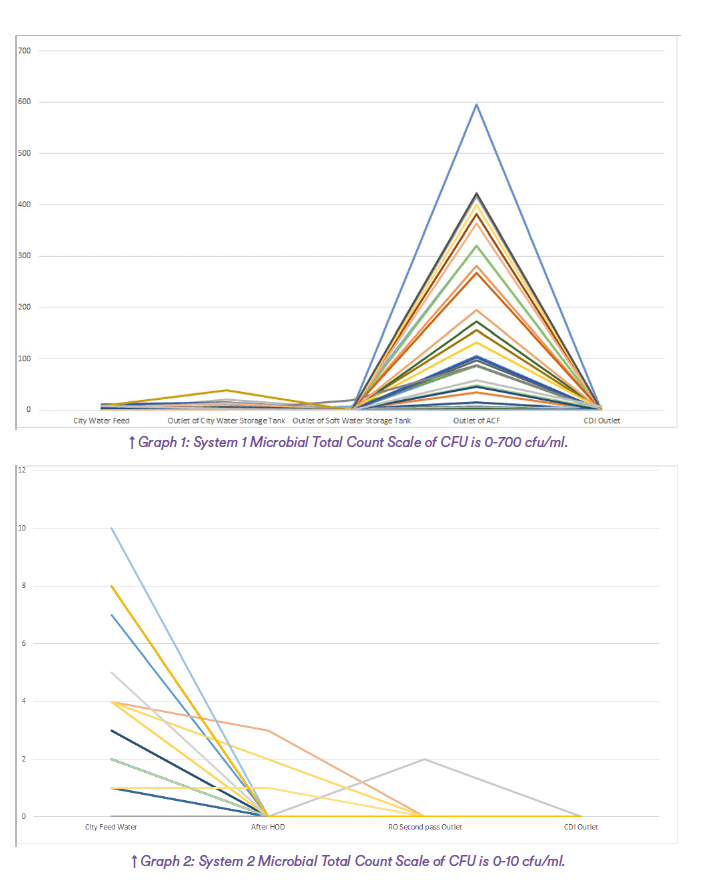
The tables record raw data from the regular monitoring of systems 1 and 2.
The graphs plot the microbial levels from the samples in the tables as a function of position in the system.
Note the very different scale of the levels of microbial contamination in the two systems, System 1 the range is from 0-600 cfu/ml and in System 2 the range
is from 0-10 cfu/ml.
From the graphs of the total count of both systems, it is easy to see that in both System 1 and System 2 the incoming water has low levels of bacteria, usually less than 10 cfu/ml. But, in System 1, as soon as it passes through the carbon filter, the bacteria grows out of control. This is the feed water for the RO membranes and must be removed by the membranes.
On the other hand, System 2 has a systematic reduction in microbiological counts from the city water inlet to the RO
feed.
System 1 has no residual biocide after the Active Carbon Filter as the residual free chlorine has been removed by the Active Carbon filter. There is a jump in the microbial levels after the water flow through the organic media of the Active Carbon Media which is exacerbated by the removal of the free chlorine. System 2 has a residual of free chlorine up to the HOD which both removes the chlorine and disinfects the bacteria at the same time.
In System 1 nearly all the micro-organisms are removed by the double pass RO to meet the PW specification. This necessitates removal of an average of
nearly 180 cfu/ml from the feed water.
In System 2 nearly all the bacteria are removed from the feed water by the HOD, the system feeds very low cfu/ml water to the RO membranes. The bacterial levels in the RO-RO system are very low, maximum 2 cfu/ml with zero at the outlet of the RO and CDI with an average of less than 0.2 cfu/ml.
System 2 demonstrates Continuous Bioburden Reduction (CBR). CBR is the ability of a system to reduce bioburden sequentially through the system components. This reduction should be achieved with minimum maintenance effort and down time. As System 2 has the ESR generating free chlorine online and the HOD disinfecting online, the reduction in bacteria is achieved by regular operation of the system.
3. Conclusions
System 1 and 2 have the same main production equipment, double pass RO. The systems are differentiated by the pretreatment feeding water to the first stage RO.
The systems perform very differently:
System 1 actively promotes bacteria growing in the Active Carbon filter and feeds the high levels to the first stage RO and all the biological load has to be removed by the membranes.
System 2 removes all the bacteria growing before the first stage RO and almost none of the biological load is removed by the membranes which are kept very clean in the process.
System 2 demonstrates Continuous Bioburden Reduction and all components of the pretreatment and production actively reduce the bacterial load heightening reliability and lowering the chance of bacteriological breaking though into the product.
Partager l’article

Keren ZALKIND ZIGELBOIM

Shlomo SACKSTEIN




