Sommaire
- Biotherapy. Médicaments de Thérapie Innovante et Annexe 1 : Analyse de son applicabilité
- How Can the Industry Drive Down the Cost of Goods to Better Serve the Patients?
- Maintaining contamination control in advanced therapy medicinal product manufacturing
- A Plug-and-Produce GMP Plant for Cell and Gene Therapy
- Maîtrise des risques : Stratégies et innovations pour sécuriser le développement des biomédicaments
- Statistical Approach to Aseptic Process Simulation: Representativeness and Proactive Alert Limit Setting for Aseptic Interventions
- Comparative Study of WFI Pretreatment Performance Electrically Based Pretreatment Outperforms Media-Based Pretreatment
- Détection à 100% des défauts critiques PP
- L’industrie pharma doit réduire sa trace carbone, ... le traitement d’air. Part 1
A Plug-and-Produce GMP Plant for Cell and Gene Therapy
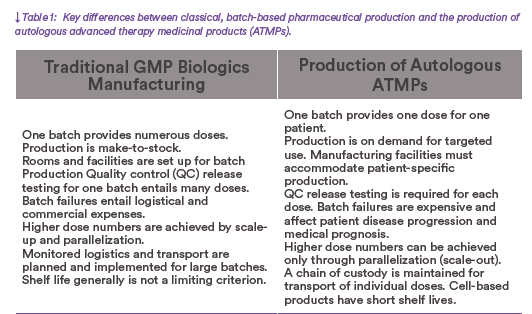
The use of approved advanced therapy medicinal products (ATMPs) remains limited despite their potential to address unmet medical needs. One example uses chimeric antigen receptor (CAR) T cells for treatment of refractory lymphoma(1). Typically, such medicinal products begin with cells that are harvested from a patient and genetically programmed to recognize and eliminate tumor cells upon reinfusion. Several cell therapies based on this and other technologies are approved for use in the United States, Europe, and China(2). Given their indications, up to 60,000 patients could be treated in those market annually. However, in 2018 and 2019 only about 1,750 patients received CAR-T treatments, and eligible recipients faced long waiting lists with manufacturers(3). Autologous use of a patient’s own blood cells has significant consequences on the manufacturing and quality control (QC) of suchtherapies. In essence, one product for one patient represents one batch — and as a result, the methods established to produce conventional drugs cannot be transferred 1:1 to autologous ATMPs (Table 1).

Case Study in Modular Facility Design and Deployment
Addressing the bottleneck
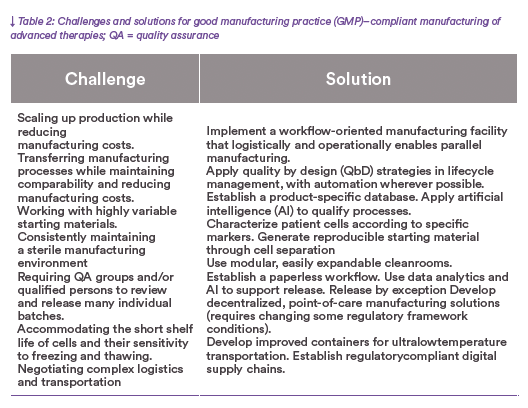
Expanding the production of ATMPs to reach more patients poses numerous challenges (Table 2).
Obstacles must be overcome at every stage of the manufac- turing process, from handling the unique starting material of each batch to accom- modating the highthroughput quality checks and complex delivery logistics for multiple batches. Autologous ATMPs are structurally complex. Any step in their manufacturing can compromise product quality, and process changes can adver- sely affect efficacy and safety. Ideally, all manufacturing and testing processes — from early development through commercial production — are scienti- fically understood and clearly defined. Good-practice (GxP) compliance is maintained throughout a product’s life cycle with controlled and easily validated process changes, production-scale changes, and technology transfers to other production sites. Also, carefully defining critical process parameters (CPPs) for production and release at an early stage — and keeping them constant over the entire life cycle of a product — greatly eases subsequent validation and qualification. Development of an ATMP should use the methodology of commer- cial production.
External conditions of production and testing should be determined simul- taneously. By defining starting materials and raw materials in advance, critical material attributes and critical quality attributes (CQAs) can be established to fulfill requirements of the quality by design (QbD) methodology(4). Thus, process transfers from development to routine production and technology trans- fers to additional production sites can be easy and controlled. This approach shortens development times from R&D to clinic, thereby significantly reducing overall costs. Here, we present a tech- nical solution designed to keep parame- ters constant, from development to routine clinical production, and enable scaleout of autologous ATMP production through parallelization.
Plug-and-produce modular manufacturing
The ISO 13485–certified CliniMACS Prodigy mobile device is used to manu- facture ATMPs(5–8). It automates all steps in patient-specific cell manufac- turing, including userprogrammable sequences for magnetic cell selection, lentiviral vector transduction of cells, CO2-controlled cell expansion, tempe- rature-controlled centrifugation, and conveyance of cells and liquids through sterile and fully enclosed tubing sets. The system is designed to introduce reagents and media safely, enable multiple appli- cations using different tubing sets, drive high process reliability, and save users time and money. Implementing a Clini- MACS Prodigy system for manufactu- ring requires a dedicated production facility. With modular design and inte- grated elements for air conditioning, lighting, fire protection, power and data cables, sensors, and supply lines, ExyCell modules simplify construction(9).
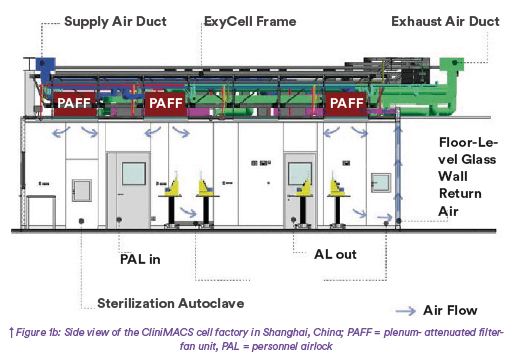
A plenum-integrated filter-fan unit (PIFF) for ceiling air return and a hyphen between plenum-attenuated filter-fan unit (PAFF) for low-level air return help a facility meet regionally different good manufacturing practice (GMP) requi- rements. Bundled utility tapping points supply process equipment with power, air, and water. The modular system balances standardization with design freedom to adapt each process environ- ment to developed production processes and optimal operations. An ExyCell module can be installed in an existing building, or a cost-effective prefabricated building can be created as the outer shell. Those complementary solutions have been integrated into a standard layout for a CAR-T production facility that provides GMP-compliant capabilities and is adap- table to user needs with minimal planning required. The overall goal is to facilitate transferring CAR-T and related cellen- gineering methods developed at univer- sities and specialized start-up companies to a current GMP (CGMP)– compliant production environment. Manufacturing capacities are made available quickly by keeping basic requirements for setting up the production environment standard.
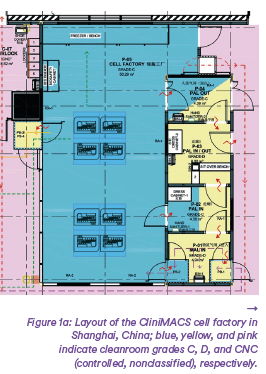
The layout is modularly expandable and includes a central production environ- ment. Support areas (e.g., warehouses, laboratories) are built adjacent to clean- rooms using standard building mate- rials (Figures 1).
Because production is functionally closed, multiple individual batches can be produced in parallel in a central cleanroom, which optimizes operating processes and helps reduce product costs. To ensure flexibility and future viability, the plant is automated as a modular “plug-and-produce” (PnP) system(10). The facility has an optimized workstation layout, with two sets of four stations and the ability to add a third set later. This layout is oriented for efficient flow and high productivity. As a biosafety level 2 cleanroom, it makes personnel and material flows unidirectional, with a sequential gowning concept from controlled, nonclassified (CNC) space to grade-C areas. All materials are brought in through active pass boxes, and all waste is sterilized through a two-door decontamination autoclave. A central supply station feeds individual worksta- tions from the ceiling, which facilitates later adjustments. Ceiling connections can be relocated with minimal impact on operations, and additional utilities can be added when needed. Final connections and terminations are cut into the ceiling; major adjustments are made by replacing a single 1.2 × 1.2 m ceiling tile using exis- ting penetrations and prewired connec- tions. The design also includes required slopes for clean media lines across the entire cleanroom area.
With this GMP plant concept, project-specific technical information such as bills of materials, prices, and schedules can be generated directly even in the early design phase. That greatly reduces commercial, scheduling, and quality risks in planning and execution. However, adjustments can be made up to the implementation phase and later, with minimal effort and impact.
A prefabricated produc-tion facility in Shanghai
To test the feasibility of the concept and examine options based on a proof of concept, a facility for clinical sample production was built in Shanghai’s ATLATL Innovation Center based on the layout described herein(12). This facility complies with CGMP regulations; meets numerous local regulations and standards for buildings, installations, and occupational health and safety; and integrates the necessary functions to produce and release ATMPs from a small footprint. The project-specific design process took two months from concept initiation to documents submission and regulatory approval. That included a redesign to create a lightweight structure version for retrofit into an existing building, a task that was simplified by the overall robustness of the concept. Designed modules were prefabricated in two weeks, tested, and packed for transport along with most of their intermediate connections (including those for clean utilities) and the corresponding commissioning and qualification documents. Modules were delivered to the construction site by conventional flat-pack transport. The PnP approach allowed for easy integration with the preconfigured infrastructure. Onsite, most connections had only to be attached to prepared supply systems, saving signi- ficant time(10). Heating, ventilation, and air conditioning (HVAC), process utili- ties, and other supporting infrastructure were provided right next to the modular plant in this project. Site preparation and installation of support structures began in the middle of December 2020, after the order for the modules was confirmed early that month. Modules were deli- vered to the site on December 26. The Chinese New Year in early February and pandemic-related delays extended the overall schedule to the end of March 2021, mostly because of lead times for delivery of wall systems and for design and installation of a dedicated qualified building management system (Q-BMS). Utilities connections were timed to prevent interference with ongoing R&D operations elsewhere in the building. After the modules and supply lines were installed, the walls were attached to the grids of the modular ceiling, and a vinyl floor was laid. Unlike a conventional modular system, the module connections are invisibly integrated into the design (Figure 2).
Structurally, the modules are supported by their own frames; they also can be suspended from an existing building structure at heights that are fully adjustable to meet process-specific requirements. Additional modules can be added at any time without interrupting ongoing production in existing modules. The onsite plant-engineering team can add or remove walls and doors as well as adjust adjacent filter-fan units. Several modifications were made before commissioning and qualification in April: integration of fully cleanable floor-level return air-duct systems into the glass walls, addition of PnP sockets, and installa- tion of fully glazed perimeter walls with 90-minute fire resistance. Plant and cleanroom qualification of GMP-critical design elements were performed in all systems with direct influence, according to defined qualification plans adapted to the plant and incorporating factory acceptance test (FAT) results where possible(11). No critical deviations were found. It is worth noting that Chinese cleanroom regulations changed at the end of 2021 to require floor air return in all plants to be classed as B or C. Thus, the original module design using PIFFs for ceiling air recirculation was adapted with a combination of recessed returnair shafts to the ceiling and a unique glass return-air shaft wherever walls are full- height glass. The floor-to-ceiling glass is advantageous in this kind of facility. Such walls are easy to clean, meet all CGMP criteria, and allow inspectors, visitors, and supervisors to view operational areas without gowning up or disrupting ongoing operations. Transparency also serves communication and rapid assis- tance in the event of an incident. Finally, high visibility encourages an organized and tidy work environment, which reduces the risk of contamination, and work quality increases when there is a line of sight to other employees and the outside world.
Paving a Path to Commercialization
Most cell and gene therapy start-ups lease space to convert into a facility for small-scale ATMP production, with the intent to build owned commercial-scale facilities after a successful market launch. The lightweight design of this PnP modular production concept is ideal for such purposes because of fast assembly, easy delivery to an existing building, and a clearance height of at least 2.7 m in a space that is only 4m high. All maintenance can be performed from the front in a mechanical room, from inside clean- rooms, or from above.
A building load of just 80 kg/m2 allows the CliniMACS Prodigy system to be installed into most buildings with minimal or no support structure. It can be supported from the floor (as in this project) or suspended from the building superstructure. Thus, this integrated solution coordinates, optimizes, and standardizes a central manufacturing process and production environment, including cleanrooms and their supplies. The modular PnP concept reduces timelines and costs of implementation while accommodating future scale-out and expansion of a GMP plant.
Rapid Deployment of a Commercial-Scale Facility
Extending the use of approved advanced-therapy medicinal products (ATMPs) to the tens of thousands of patients who could benefit from such treatment requires a 10- to 100-fold production scale-up. Given that each autologous ATMP batch yields one dose for one patient, expanding production throughput is not a question of boosting volume, but rather of amplifying single manufacturing runs. That is, scale-up is actually scale-out, and the dimensions of the ensuing endeavor extend beyond what occurs in the cleanroom.
Coupled with each production run is a battery of controls for raw materials, in-process conditions, and product release. Higher throughput requires a smart facility layout that ensures segregated and controlled flows of materials, people, waste, and products. Increasing the number of processed batches by several times requires stringent process monitoring and validation based on expanded data pipelines.
The plug-and-produce (PnP) cell-manu- facturing facility is based on two core technologies: the ISO 13485–certified CliniMACS Prodigy mobile device and the patented ExyCell preconfigured and prefabricated facility modules. The integrated design is a ballroom- concept good manufacturing practice (GMP) “cell factory” for one to a dozen CliniMACS Prodigy instruments that enables fast- tracked construction, streamlined quali- fication, and modular expansion.
The pilot deployment demonstrated the relatively short timeline and reduced cost of installing an integrated CliniMACS Cell Factory facility. Furthermore, this plant has confirmed that the PnP concept standardizes cell manufacturing processes, production environments, and supplies. Thus, high-quality GMP CliniMACS Cell Factory production units provide building blocks for scaling out ATMP production capabilities. Based on such a modular expansion strategy, routine production of 1,000 batches per year (about 22 batches per week), for example, would entail the installation of two ballrooms with 24 CliniMACS Prodigy instruments each and covering a combined surface area of about 200m2.
Enhancements for Scale-out
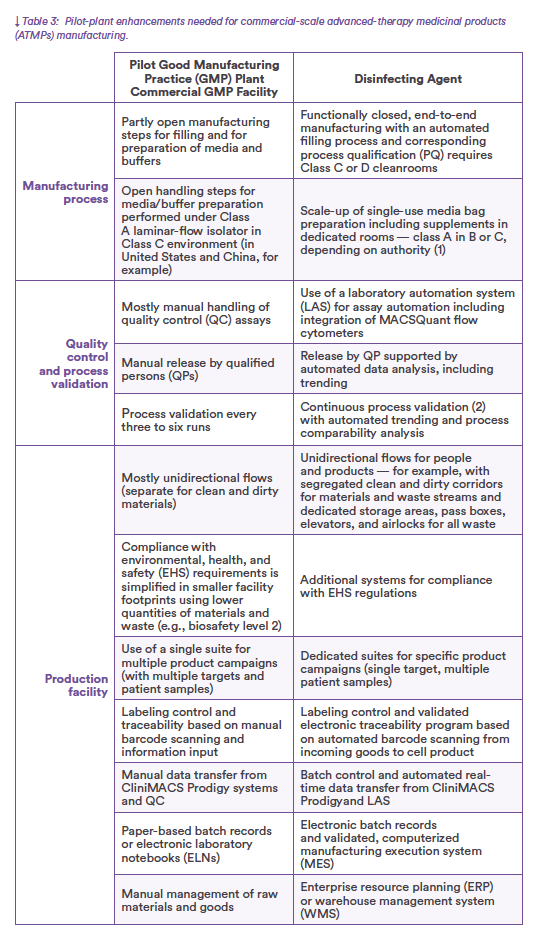
Evaluating the pilot GMP plant in the context of a commercial-scale facility highlights three areas for enhancement: manufacturing process modifications that completely integrate and automate the process from starting material to final filling; integration and automation of quality control and product release; and production facility adaptations that guarantee segregated and reproducible flows, secure traceability, and automated data handling (Table 3).
The CliniMACS Prodigy platform is a closed, sterile system that automates every step of patient-specific cell manufacturing. Each system houses an end-to-end manufacturing process with controlled inputs and predefined outputs, which greatly simplifies the layout of a ballroom housing multiple CliniMACS Prodigy workstations, each station dedicated to a production campaign.
At commercial scale, the end-to-end manufacturing process features media and buffer supply in single-use bags for each production batch. Stockpiling, movement, and use of those bags — along with all other materials entering and leaving the cleanroom — come through an enterprise resource planning or warehouse management system that collects barcoded information. Automation-enabled devices and real- time data transfer for production moni- toring, quality analytics, and product release are essential to commercial-scale manufacturing. Components of the necessary infrastructure include a labo- ratory automation system (LAS) with integrated MACSQuant flow cytome- ters for carrying out quality control (QC) assays from samples to results. Real-time data transfer from CliniMACS Prodigy instruments facilitates conti- nuous process monitoring and electronic batch control. A computerized manufacturing execution system (MES) with electronic batch records monitors campaign-wide processing. The MES covers the complete manufacturing process from sample materials to formulated and finished final drug products. ExyCell modules meet many layout requirements for commercial-scale production facilities. Approaches to airflow management, execution quality, and qualification remain the same as those for the pilot GMP plant. Adaptations address the needs of a larger facility that operates with nonexpert personnel (e.g., technicians) and processes greater quantities of materials and waste than the smaller pilot operation. The design secures segregated and reproducible flows of materials and waste while opti- mizing the arrangement and use of support areas around the core produc- tion environment. Appropriate systems ensure compliance with environmental, health, and safety regulations — e.g., fire protection, emergency exit routes, and waste disposal.
A turn -key commercial facility
The CliniMACS Cell Factory platform complemented by ExyCell modules is a flexible, rapid-deployment solution to facilitate scale-out of ATMP production. With end-to-end closed processing, increasing throughput entails simply adding more CliniMACS Prodigy works- tations to a campaigndedicated suite. The reproducible layout and supply concepts simplify scale-out to a modular expansion in which integrated systems track materials flow, control manufac- turing, and automate QC. Furthermore, planning experience and systematic documentation from suppliers provide for construction and qualification of a practically turn-key facility in months, ultimately shortening the time to market for promising autologous therapeutics.
References
1. PE 009-16. Annex 2A. Manufacture of Advanced Therapy Medicinal Products for Human Use. Guide to Good Manufacturing Practice for Medicinal Products. PIC/S Secretariat: Geneva, Switzerland, 1 February 2022; https://picscheme.org/docview/4590.
2. Ares1380025-30/03/2015. Annex 15:Qualification and Validation. EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. European Commission:Brussels, Belgium, March 2015; https://health.ec.europa.eu/system/files/201611/2015-10_ annex15_0.pdf.
3. Bennett C. CAR T-Cell Therapies: Early Insights into Access and Affordability. ObR Oncol. 11(6) 2018; https://obroncology. com/article/ car-t-cell-therapies-earlyinsights- into-access-and-affordability.
4. ICH Q8(R2). Pharmaceutical Development. US Fed. Reg. 71(98) 2009; https://database.ich.org/sites/default/files/ Q8_R2_Guideline.pdf.
5. Apel M, et al. Integrated Clinical Scale Manufacturing System for Cellular Products Derived By Magnetic Cell Separation, Centrifugation and Cell Culture. Chem. Ing. Tech. 85(1–2) 2013: 103–110; https://doi.org/10.1002/cite.201200175.
6. Kaiser AD, et al. Towards a Commercial Process for the Manufacture of Genetically Modified T Cells for Therapy. Cancer Gene Ther. 22(2) 2015: 72–78; https://doi.org/10.1038/cgt.2014.78.
7. Mock U, et al. Automated Manufacturing of Chimeric Antigen Receptor T Cells for Adoptive Immunotherapy Using CliniMACS Prodigy. Cytother. 18(8) 2016: 1002–1011; https://doi. org/10.1016/j.jcyt.2016.05.009.
8. Jackson Z, et al. Automated Manufacture of Autologous CD19 CAR-T Cells for Treatment of Non-Hodgkin Lymphoma. Front. Immunol. 7(11) 2020: 1941; https://doi.org/10.3389/fimmu.2020.01941.
9. Mussati L, et al. Ceiling Module for the Construction of a Clean Room. US Patent Application 16/512,451, filed 21 January 2021
10. Jebari A, et al. Planung und Realisierung von Modularen Plug und Produce- Systemen. TechnoPharm 11, 2021: 72–78.
11. Pharmaceutical Engineering Guide for New and Renovated Facilities: Commissioning and Qualification, Volume 5, Second Edition. ISPE:North Bethesda, MD, June 2019; https://ispe.org/publications/guidance-documents/baseline-guide-vol-5- commissioning-qualification- 2nd-edition.
12. Kappeler SR, et al. Realisierung einer Standardisierten GMP
Anlage zur Herstellung von Personalisierter Medizin. TechnoPharm 11, 2021: 184–193; https://www.ecv.de/beitrag/ TechnoPharm/ Realisierung_einer_ standardisierten_GMP-Anlage_zur_ Herstellung_ von_personalisierter_Medizin. Pharmaceutical Inspection Co-operation Scheme (PIC/S). (2023). Annex 2A: Manufacture of advanced therapy medicinal products for human use. In Guide to Good Manufacturing Practice for Medicinal Products Annexes (PE 009-17). Retrieved from PIC/S website.
Partager l’article