Sommaire
- Vaccines & Endotoxin, a Challenging World.
- Méthode alternative d’identification. Exemple d’une implémentation d’un système Maldi Tof au laboratoire de contrôle.
- Implantation d’une méthode microbiologique alternative et polyvalente au laboratoire. Exemple du BACTALERT 3D®
- From Variable Operator Numeration To The Standardized 3P® Station Automated Colony Counting On Environmental Monitoring Culture Media Plate
- Analyse de la charge microbienne en temps réel pour prévenir les risques de contamination et mieux contrôler les procédés.
- ISO 22519 PW/WFI production systems standard: Quality aspects.
- Eau pour préparations injectables en vrac (productions, coûts). Étude de cas axée sur la qualité microbiologique.
- Coming to basics on rouging.
- Winning combination: Reducing particles in RTU packaging by aligning lid material, adhesive and sealing parameters in a holistic approach.
Winning combination: Reducing particles in RTU packaging by aligning lid material, adhesive and sealing parameters in a holistic approach.
Sterile injectable drugs play a crucial role in the treatment of disease. The global sterile injectable drugs market was valued at approximately USD 369.7 Bn in 2017 and expected to grow to around USD 779.9 Bn by 2024(1). All stakeholders in this industry have the inherent goal to make injectable drugs safe and effective. This includes an expectation that every lot be essentially free from visible particulates, as described in various USP chapters and pharmacopeias(2), and that manufacturers strive to minimize all particulate and foreign contamination(3).
Numerous guidance documents assist manufacturers in achieving quality, efficacy, and adherence to safety standards. However, there is currently no road map to achieving drugs that are essentially free from foreign matter. With all the discussions since the first PDA/FDA Glass Quality Conference in May 2011, there are still product recalls related to visible particulate matter(4, 5). These one off failures (a single particle) still have resulted in drug manufacturers pulling entire lots of drugs from the market sacrificing commercial interests, for patient safety and regulatory scrutiny. These decisions have resulted in drug shortages. At the time of this case study, there are 123 drugs on the US FDA’s drug shortage list(6).
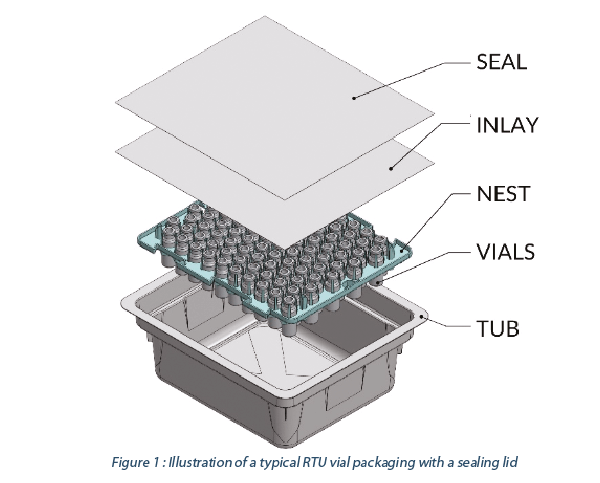
Primary packaging (the container/closure) is one of the lead sources of “visible” particles in parenteral drugs(7). Ready-to-use (RTU) components are delivered in a so-called nested configuration, which prevents glass-to-glass contact and reduces defects like scratches, breakage, and particles. These packaging materials are an additional source of foreign contamination.
In this paper we focus on the sealing lid as one source for particles in RTU containers. We believe we can achieve improved particle control by matching lid material, adhesive, and processing parameters (Part I), and subsequently we will discuss five focus areas for pharma manufacturers and their suppliers to further reduce particle risk (Part II).
1. Lids as a source of particles in RTU containers
The application of ‘peelable’ adhesives to the roll stock and die-cutting may result in loose fibers and/or adhesive artifacts as the tub/tray sealing lid is in immediate proximity to, and occasionally in direct contact with the nested containers/ closures. High peel forces may further stress these materials leading to additional contamination risk (7). Selecting the right combination of lid material and adhesive, along with strict process controls, can influence the performance of the entire RTU packaging system.
To increase the practical benefit of this case study, we concentrate on materials known under the brand name DuPontTM Tyvek® – hereinafter referred to as sealing material or lid material. It is, to our knowledge, the most common sealing material for this purpose and used by all major suppliers of RTU containers. We want to point out that DuPont was neither involved in the testing nor the creation of this paper. However, we do want to underline how collaboration along the supply chain, including lead pharma customer(s), helps to reduce the risk of visible particulates significantly.
Study design
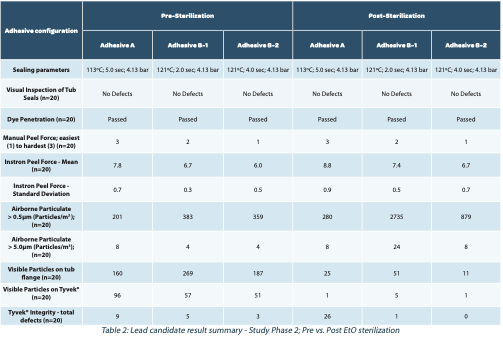
We evaluated six different sealing material/adhesive combinations, both hot melt and water-based, in standard, commercially available configurations. More precisely, the combinations were tested on the SCHOTT iQ® RTU Platform, which comprises pre-filled syringes, vials, and cartridge packaged in the industry-standard nest & tub (ISO 11040-7) configuration.
Seal qualification was performed for all study materials before initiation to optimize sealing parameters: time, pressure, and temperature. This optimization included studies to achieve the minimum required peel force while also leading to a better understanding of influences on adhesive migration into the porous material – which can lead to a thinner layer of adhesive at the material interface – and less variation among all four sides of the applied seals. This has resulted in lower peel force standard deviation, reduced adhesive migration, a cleaner visual transfer which is free from bubbles, gaps, channels and/or delamination, and better seal integrity as tested by dye ingress.
Forty tubs of each format were sealed and tested both pre and post standard Ethylene Oxide (EtO) sterilization to evaluate the impact, if any, of the sterilization cycle on critical product attributes. It must be noted that each filling line manufacturer performs delidding in a slightly different manner, i.e., peeling in multiple directions or heating to soften or melt the adhesive, to name a few. In this evaluation, we simulate what we consider a worst-case condition: ambient temperature, low angle, single direction, manual peeling.
Materials and Equipment
• 1073B Tyvek® with various adhesives
• Polystyrene (HIPS) injection molded tub
• Microscope Olympus SZX-10 Stereo microscope equipped with Infinity Camera
• Contact adhesive roller
• Instron 5542A single testing column system
• Hach Met-1 3400 series particle counter.
After a minimum hold time of two weeks post-processing each configuration was subject to the following testing:
1. Seal strength (Instron Peel force in Newtons). Five tubs, 4 coupons per tub (n=20), 25mm in width were cut and evaluated. Max, min, mean, and standard deviation were compared for each iteration.
2. Peel force (manual). Twenty samples each were evaluated for consistency peel forces across the entire seal and visually inspected according to ASTM F 1886/F1886M-09 Test method for determining the integrity of seals for flexible packaging by visual inspection.
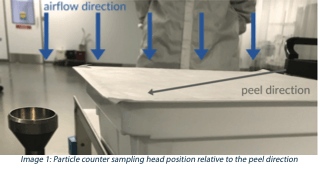
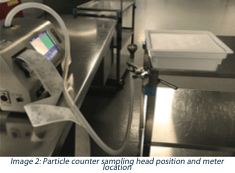
3. Shed airborne particulates during peeling (non-viable particle counts). The work surface was pre-cleaned with a contact roller and other materials removed except the tub being tested and the particle counter. Lid peeling was performed at ambient temperatures in an ISO-7 environment to better control the air velocity during sampling. The peel was slow (5-6 seconds) using even pressure at a low angle (15-20 degrees) in a single direction (diagonally) across the seal surface towards the sample head. The sample head was positioned 30mm below the tub flange. A 6-second delay/purge was followed by a 20-second sample collection at the maximum sampling rate of 1.77 ft3/min. Particle counts were recorded, noting the average and standard deviation for both the 0.5 μm, and 5μm size bins.
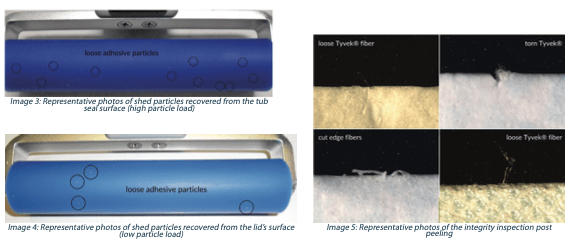
4. Loose particulate on both the tub seal and the lid material (adhesive side only) – Visual inspection of adhesive residues on the tub seal surface post peeling. A head-to-head comparison of the potential for shed particles was accomplished utilizing a contact adhesive roller to evaluate loose particles resulting from adhesive residues and strain/tearing of the sealing lid. The roller lifts particles off the tub seal surface and the lid directly. The number of collected particles were compared for each format.
5. Microscopic inspection 10-63X of the Tyvek® post peeling.
For each configuration, a microscopic inspection was performed on the edges of five (5) lid sheets. Photos were taken of any loose fibers, tears, or related structural defects and formats compared.
Image 3 and 4 represent labile particles isolated from the tub surface and the lid (adhesive side only) post peeling. Using this technique, labile particles can be sized, quantified, characterized, and contrasted.
Discussion
From the data, we draw the following conclusions:
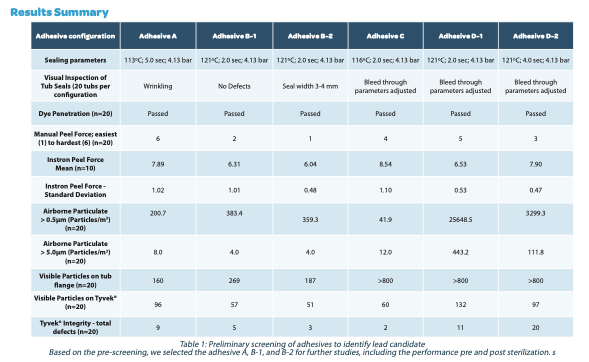
• Various attributes were weighted, and the lead candidate was identified as adhesive A.
• Peel forces (>10N) increased by an average of 12% after EtO sterilization.
• Shed airborne particles >0.5μm for one iteration in particular (B-1) increased >7x after sterilization.
• Higher peel forces were correlated with lower shedding of airborne particles and lid damage.
• Loose particles on the tub flange and seal surface decreased significantly with EtO sterilization. This may be due to the cross- linking of the adhesive by the sterilization process. Further study is required.
• Loose particles were higher on the tub seal surface when compared to the lid post peeling. This poses a problem as the lid is typically discarded after removing while the tub may index deeper into the filling operation when used as secondary containment for the vials.
Based on these findings, further opportunities for improvement were identified and subjected to further studies. The results of these studies are pending.
2. Five focus areas to tackle particle risk
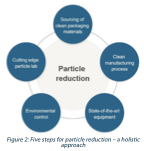
The approach behind these results, which led to reducing the particles generated from the lid material, can be structured in five parameters. In this case study, it was applied at an RTU container supplier, but it could easily be applied to a pharmaceutical manufacturing environment as well. Figure 2 outlines the five parameters.
This holistic approach to particle reduction includes sourcing of clean components or packaging materials. When looking at all potential sources of contamination, it is much more effective to collaborate with suppliers that also have controls in place. It helps to understand the capabilities and the manufacturing environment for all incoming materials. Suppliers of non-shedding equipment (cleaning materials, gloves, gowning supplies) or trace-clean contact materials (transfer tubing, HEPA filters, sterilizing filter membranes, housings) will allow the drug manufacturer to focus on utilities, process, and product contact surfaces within the filling suite.
With regards to the RTU packaging itself, it is advantageous for the pharma company if their supplier follows a platform approach since this will lead to more predictable particle scenarios. For example, the SCHOTT iQ® platform uses the industry-standard tub (ISO 11040-7) for all RTU containers. This harmonization allows the use of standard components like the same inlay and lid material across product groups.
Next, special attention is given to a clean manufacturing process and the respective equipment. Legacy production equipment may generate and spread source contamination (belts, accumulation tables, etc.), so state-of-the-art equipment should be evaluated for the potential to reduce particles further.
Understanding the sources of particles and implementing effective controls in production and packaging areas will further reduce contamination. Tight environmental monitoring is an additional feedback loop required for success. In this context, it is important to understand that bioburden control programs do not necessarily double as particle control programs. Individual components must rather be tested ’as received’ and verified lot-to-lot as non-shedding. Otherwise, in-house cleaning programs may be required.
All these steps can be augmented only with robust capabilities in lab equipment, isolation techniques, and expertise for particle analysis. For example, in-house forensics lab capabilities are required to isolate, quantify, identify and mitigate sources of contamination. Developing a master reference library (FTIR/ATR; SEM-EDS and elemental) for all materials introduced into the filling suite makes tracking down the source(s) much more efficient. The finished product can tell what is wrong, but it is always too late to do something about it.
There are many opportunities to reduce sources of extrinsic contamination. Following a holistic approach and cooperating closely along the value chain can lead to true innovation in contamination control programs. Eventually, this will bring the pharma industry closer to its goal of becoming ’essentially free from visible particles.
Partager l’article

John BERGGREN – SCHOTT AG
John Berggren joined SCHOTT 2 years ago after 25 years in pharmaceutical manufacturing (tablets and injectables) where he managed quality, analytical laboratories, drug/device development, supplier quality, engineering and technical operations. In his current role, he directs contamination control programs for the SCHOTT iQ® platform.
john.berggren@us.schott.com

Gregor DEUTSCHLE – SCHOTT AG
Gregor Deutschle started with SCHOTT in 2008 and has more than 7 years of experience in parenteral packaging, ready to use containers and fill & finish processes. He held various positions and is now the Director Business Development Sterile Solutions for the adaptiQ® ready to use vials. He focuses on accelerating the adoption of ready to use packaging and advancing aseptic manufacturing in the pharmaceutical industry. Gregor holds a degree in mechanical engineering.
gregor.deutschle@schott.com
Références
1. Zion Market Research (2019): Global Sterile Injectable Drugs Market https://www.zionmarketresearch.com/news/sterile-injectable-drugs-market [26th May 2019] 2. U.S. Pharmacopeia (2014): USP Chapter [1] Injections, <790> Visible Particulates in Injections, USP 37/NF 32.
3. PDA Journal of Pharmaceutical Science and Technology (2015): Industry Perspective on the medical risk of visible particles in injectable drugs products, Vol. 69, No.1
4. Journal of Pharmacovigilance (2014): Particulate Matter in Injectables: Main cause for Recalls, Vol. 3:1 / https://www.longdom.org/open-access/particulate-matter-in-injectables- main-cause-for-recalls-2329-6887-3-e128.pdf [26th May 2019]
5. Laguna Treatment Hospital (2019): Examining FDA Drug Recalls and Adverse Reactions, https://lagunatreatment.com/fda-drug-recalls/, [26th May 2019]
6. PDA (2018): FDA is Advancing New Efforts to Address Drug Shortages, https://www.fda.gov/news-events/fda-voices/fda-advancing-new-efforts-address-drug- shortages [26th May 2019]
7. FDA.(2019): Archive for Recalls, Market Withdrawals & Safety Alerts. https://www.fda.gov/ safety/recalls-market-withdrawals-safety-alerts/archive-recalls-market-withdrawals-safety- alerts [26th May 2019]