Sommaire
- Vaccines & Endotoxin, a Challenging World.
- Méthode alternative d’identification. Exemple d’une implémentation d’un système Maldi Tof au laboratoire de contrôle.
- Implantation d’une méthode microbiologique alternative et polyvalente au laboratoire. Exemple du BACTALERT 3D®
- From Variable Operator Numeration To The Standardized 3P® Station Automated Colony Counting On Environmental Monitoring Culture Media Plate
- Analyse de la charge microbienne en temps réel pour prévenir les risques de contamination et mieux contrôler les procédés.
- ISO 22519 PW/WFI production systems standard: Quality aspects.
- Eau pour préparations injectables en vrac (productions, coûts). Étude de cas axée sur la qualité microbiologique.
- Coming to basics on rouging.
- Winning combination: Reducing particles in RTU packaging by aligning lid material, adhesive and sealing parameters in a holistic approach.
Endotoxins & Vaccines, a Challenging World.
Endotoxins are heat – stable lipopolysaccharides from Gram-negative bacteria and are potential contaminants that can be introduced during the manufacturing of pharmaceutical products, including vaccines. Parenteral pharmaceutical products undergo endotoxin testing because endotoxins are pyrogenic in humans and can induce severe physiological reactions. Currently, animal-derived Limulus amoebocyte lysate (LAL) assays are widely used. Assays using recombinant Factor C (rFC), a non-animal-derived reagent, have been proposed as an alternative and since 2020 rFC is considered as a compendial test in Europe. Endotoxin
Endotoxins are heat-stable lipopolysaccharides from Gram-negative bacteria and are potential contaminants that can be introduced during the manufacturing of pharmaceutical products, including vaccines. Parenteral pharmaceutical products must undergo endotoxin testing since endotoxins are pyrogenic in humans and can induce severe physiological reactions. Currently, animal-derived Limulus amoebocyte lysate (LAL) assays are widely used. Assays using recombinant Factor C (rFC), a non-animal-derived reagent, have been proposed as an alternative. Recently in Europe, in June 2020, rFC was introduced in a general Chapter as compendial assay that will be effective on 01 January 2021.
1. Pharmacopeial environment around endotoxins
Within the last 10 years, there have been developments in the regulatory significance of endotoxin testing. The change is mainly due to the context around the Low Endotoxin Recovery (LER) phenomenon, which is still debated since 2013, as well as the focus on alternative methods to measure endotoxins with rFC (and full recombinant). Even though commercial reagent solutions exist now for more than 15 years, the rFC-based method is gradually becoming a compendial method.
The first to approach the rFC from a regulatory point of view was the United States Pharmacopoeia (USP) with the publication in the Pharmacopeial Forum (PF) of a stimuli article from Lonza in 2010. Generally, a stimuli article is written as a pre-draft for a future text, but in that case, it was never published in the USP as rFC was still single vendor technology at the time. Then, in 2012, the Food and Drug Administration (FDA) published the guideline Endotoxins and Pyrogens Questions & Answers to clarify and replace the former Guideline LAL for the final product (published in 1987), in which the FDA specifies that an alternative method to LAL based on rFC can be implemented if it is duly validated and proven. Such alternative procedures and methods should be validated as described in the USP General Chapter <1225>, Validation of Compendial Procedures, and should be shown to achieve equivalent or better results.
Finally, since 2013, new conferences in the USA and Europe have started to flourish based only on the specific context of pyrogens and bacterial endotoxins as well as the introduction and implementation of rFC.
We are going to take a detailed look at the advancement and the evolution of regulatory texts concerning endotoxins and specifically the recombinant factor C in four essential and influential pharmacopoeias: the European Pharmacopoeia, the United States Pharmacopoeia, the Japanese Pharmacopoeia, and the Chinese Pharmacopoeia.
– European Pharmacopeia (Ph. Eur.): In 2015, the Ph. Eur. added a statement in chapter 5.1.10 “Guideline for using the test for bacterial endotoxin” to allow the use of rFC as an alternative to the LAL and at the same time initiated a discussion with the PDG (Pharmacopeial Discussion Group) on the possibility of including a seventh method “G” using the rFC reagent in the harmonized chapter 2.6.14. But it was still too early in 2015 to consider the rFC as a compendial test. However, in 2017 a request from a stakeholder regarding the possible development of a chapter on Bacterial Endotoxin Test (BET) using rFC prompted the Ph. Eur., to resume the work started in 2015; it was then considered that the scientific data and knowledge accumulated in the meantime, the number of kits available in Europe, would allow the development of such a chapter. The Ph. Eur. commission decided at its 160th session to develop a separate chapter entitled “Testing for Bacterial Endotoxins using Recombinant Factor C” new Chapter 2.6.32. The draft of this new Chapter 2.6.32. has been published in Pharmeuropa 31.1 for public inquiry (from end of December 2018 until end of March 2019). This new Chapter 2.6.32 describes a BET performed using a rFC-based method on the horseshoe crab gene sequence to quantify endotoxins, as well as an endpoint fluorometric detection method. Ph. Eur. would like to point out that no special sample preparation (e.g. phage ligand) is described. Only the fluorometric method is included in this Chapter because this is the read out used in all rFC kits currently available on the European market and in most scientific data. This Chapter could be revised to include the full recombinant approaches i.e. rFC chromogenic method developed by a third Japanese supplier (i.e., PyroSmart kit). In addition to this new Chapter, to promote acceptance of the method, Ph. Eur. will soon launch a collaborative study to verify rFC methods on different products and compare them with classical LAL methods. This new Chapter was officially published in the edition 10.2 and will be effective in edition 10.3 January 2021. In parallel, Ph. Eur. revised the guideline 5.1.10. to be also published in edition 10.3 to precise the use and implementation of this new Chapter 2.6.32.
– United States Pharmacopeia (USP): End of January 2018, the USP published in the PF 44(4) a new Chapter in draft proposal <1085> “Guidelines on endotoxins test” with the justification of the retirement of the 1987 FDA (1) guideline, where a lot of useful information was removed. This new 18-page guide focused primarily on the LAL test and its environment. First, a very small section mentioned alternative tests to the LAL with the rFC but was revised 2 years later with the sub-Chapter <1085.1> “Use of Recombinant Reagents in the Bacterial Endotoxins Test – Photometric and Fluorometric Methods Using Recombinantly Derived Reagents” (details on this sub-Chapter are given later in this section). The same year, rFC was approved by FDA as endotoxin release method for Elli Lilly’s Emgality™ drug (Galcanezumab)). Then, USP organized a specific Workshop on 10 and 11 June 2019 at Rockville, “Future of Endotoxins and Pyrogen Testing: Reference Standards and Procedures”. A specific session was dedicated to examining the implementation of alternative test methods for endotoxin testing (Session 2: Alternative Methods: Is an Alternative Source of Factor C Desirable?). Following this workshop, the USP, to the great surprise, released a revision of the harmonized Chapter <85> for public comment in PF 45 (5) August 2019 that allowed the use of recombinant reagents as an alternative to LAL. However, based on stakeholder feedback, this revision proposal was canceled in April 2020. Stakeholders recommended the development of an independent Chapter rather than inclusion in a harmonized Chapter. This was the sub-Chapter <1085.1>. This sub-Chapter includes recommendations for the use and qualification of recombinant reagents as alternatives to LAL reagents. It also provides guidance on Quality Assurance oversight of recombinant reagents for BET, which are currently not regulated by the FDA. rFC remains allowed as an alternative method, and its target publication in PF 47 (1) is January-February 2021. In parallel, in May 2020, USP accepted for publication in the PF, a new stimuli article entitled “Application of Recombinant Factor C Reagent for the Detection of Bacterial Endotoxins in Pharmaceutical Products and Comparability to Limulus Amebocyte Lysate” written by Jay Bolden. This article can be commented until the end of July 2020.
– Japanese Pharmacopeia (JP): The JP Expert Committee on Biological Methods has launched a kick-off discussion to develop a BET using the reactive rFC for inclusion in the JP General Information. When this draft is available in English, the JP will send it for public consultation to Ph. Eur. and USP. JP experts intend to harmonize the use of rFC reagent in the future through the CPG. JP believes that sharing information in detail is important for future harmonization. JP has launched two collaborative studies in Japan on the comparison of LAL and rFC (one in 2017, one in 2018, but still in the Japanese language). JP is thinking of an rFC Chapter in 2021 (JP 18th Edition) but wants to work with USP and Ph. Eur. for future harmonization. Finally, in September 2019, Pharmaceuticals and Medical Devices Agency (PMDA) has released a new text to be commented entitled “Bacterial Endotoxins Test and alternative methods using recombinant protein- reagents for endotoxin assay”. In this new text, it is mentioned “… recombinant protein-reagents for endotoxin assay have been developed as alternatives to lysate reagents for the purpose of protecting horseshoe crabs, ensuring a stable supply of reagents, reducing differences between reagent lots, and improving the continuity of the tests…”
– Chinese Pharmacopeia (ChP): The endotoxin testing is described in the ChP version 2015 in Chapter 1143, “Tests for Bacterial Endotoxin”. This Chapter describes Tachypleus Amebocyte Lysate (TAL) method with a gel clot (limit test and semi-quantification test) as well as photometry methods. There is no description or recombinant reagent in this Chapter. However, ChP has proposed in 2019 for the future ChP 2020 expected by June 2020 in the Chinese language a new Chapter entitled « Guideline for the Application of the Test for Bacterial Endotoxin ». In this new guideline, there is a description of new bacterial endotoxin test methods that have been successively developed to meet the needs of bacterial endotoxin test for particular products or to reduce the use of TAL reagent. Examples include the rFC method with a specific annex.
2. Endotoxin testing in the vaccine industry
Bacterial endotoxin testing assays are used for four different purposes: manufacturing process monitoring testing (MPMT), in-process control testing (IPCT), final bulk, or product release (including raw material) and environmental water testing.
For the first two purposes, endotoxin testing ensures the reduction or elimination of endotoxins originating from raw materials and/or host cells during the manufacturing of biological products. In turn, the final product release testing ensures product safety by ensuring that the defined monography endotoxin limits of the said product have been reached. Endotoxin water testing is also critical to ensure consistently high-quality water used for equipment cleaning, and ingredient water or raw material to all pharmaceutical processing. A related area is with depyrogenation studies.
There is no particularity linked to final product/raw material or environmental water endotoxin testing in the vaccine industry 4 Microbiologie 12 I La Vague N° 66 I Juillet 2020 compared to others. However, when it comes to the testing of intermediate stages during the production of biological products involving Gram-negative bacteria, endotoxin testing can be challenging. This is particularly true when producing:
• A bacterial vaccine based on a Gram-negative bacterial antigen such as our Vi capsular polysaccharidic Typhim Vi® vaccine based on purified Vi polysaccharide from Salmonella enterica serovar typhi (http://products.sanofi.ca/fr/typhim.pdf)
• A conjugate vaccine based on Gram-negative toxoid protein, outer membrane vesicle or protein complex as protein carrier such as our Menactra® vaccine containing each of meningococcal A, C, W, and Y polysaccharides conjugated to diphtheria toxoid protein carrier (https://www.fda.gov/vaccines-blood-biologics/vaccines/ menactra)
• The recombinant proteins using Gram-negative bacteria as host cells such as Escherichia coli
When vaccine antigen or protein carrier is derived from Gram-negative bacteria, endotoxins are inherent in the product per se. They are an inevitable part of the vaccine production and are considered as process derived substances. In that case, BET can be used to monitor process consistency, characterize the endotoxin content in the product, and depending on the product type, can be associated with a pyrogen test to release the final product.
Historically, endotoxin methods were developed to detect low amounts of endotoxin in parenteral solutions administrated in large volumes, such as saline infusions. Sensitive detection of endotoxin was critical.
Today, the challenge that we face is not a sensitivity issue but rather being able to satisfactory recover the positive product control (PPC) spike. The difficulty is exacerbated by the Gram-negative bacterial nature of our antigens or host cells and the use of different treatments in purification steps during new process development or optimization where sample formulation has not been set yet.
The BET has become an essential tool for developing our processes with an increased focus on quality by design and process control. Depending on vaccine families as detailed above, levels of endotoxin can be much higher than classical parenteral solutions (e.g., insulin or viral vaccines).
Indeed, for culture intermediate stages (upstream stages), levels of endotoxin can reach up to 1E9IU/mL. For more purified stages (downstream stages before drug substance), reduction or elimination steps are implemented to reduce endotoxin content to an acceptable level, and samples are often more inhibitory than the final product.
For these complex samples, especially for purification intermediates, LAL-based methods can present some drawbacks due to the LAL enzymatic cascade being potentially subject to interferences. Interferences can come from the complex culture medium and activate the alternative non-specific endotoxin LAL (Factor G) pathway but also from chemicals used during the manufacturing purification process, product exposition to cellulose filters, etc.
By exploring the different interferences from our processes, we found that the use of rFC based methods was an appropriate solution to overcome some of the Kinetic Chromogenic LAL assay ’s difficulties.
Below are two different examples where endotoxin testing was used to quantify high endotoxin concentrations and where rFC performances were identical or better than the Kinetic Chromogenic LAL test for MPM and IPC samples.
For finished products or environmental water samples, the demonstration that both Kinetic Chromogenic LAL and rFC assays are adequate for testing will not be shown in detail in this article.
Rapidly, we compared two Kinetic Chromogenic LAL- and two rFC-based assays for endotoxin detection in four complex human vaccine matrices (3 viral vaccines and 1 bacterial vaccine potentially presenting natural endotoxin content from the Gram-negative antigens it contained). We showed that the results for the rFC-based assays were at least equivalent to those for LAL. The rFC-based assays were found to be adequate but slightly less suitable for one of our products which contained proteases and the methods used to inactivate the proteases reduced the assay performance. However, samples containing proteases that can liberate the coloured substrate or fluorophore in the absence of endotoxin are problematic for both the LAL-based and rFC-based endotoxin assays. Likewise, LAL was adequate but less suitable for another product which contained glucans (please refer to the paper Marius et al., 2020 in the PDA Journal for more details). In a second study, we demonstrated that the performance of the rFC-based method was at least similar to that for the Kinetic Chromogenic LAL-based methods for the detection of endotoxin in water samples in our setting (water for injection, purified water and cleaning validation samples; publication on-going).
a. Example of high endotoxin concentration from drug substance derived from Gram-negative bacteria manufacturing process
As stated above, endotoxin test methods were first developed to detect minute quantities of endotoxin.
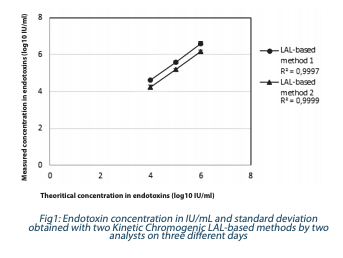
In this example, samples were diluted in endotoxin-free water to target theoretical concentrations of 107 IU/mL, 106 IU/mL and 105 IU/mL, and the exact same dilution tube was tested by two different endotoxin methods (two Kinetic Chromogenic LAL-based methods). Each concentration was tested on three different days by two different analysts. Results (concentration and standard deviation) are shown in Figure 1.
High dilutions were applied to samples to fit the standard curve (50 4 Measured concentration in endotoxins (log10 IU/ml) Theoritical concentration in endotoxins (log10 IU/ml) Fig1: Endotoxin concentration in IU/mL and standard deviation obtained with two Kinetic Chromogenic LAL-based methods by two analysts on three different days Measured concentration in endotoxins (log10 IU/ml) Theoritical concentration in endotoxins (log10 IU/ml) Fig2: Endotoxin concentration in IU/mL obtained with two Kinetic Chromogenic LAL-based methods and one rFC-based method Microbiologie Juillet 2020 I La Vague N°66 I 13 – 0.05 IU/mL) and to obtain appropriate PPC recovery (~100%). Error on endotoxin quantification should be high since essential dilutions were prepared (dilutions up to 106 in endotoxin reagent water), but excellent correlation coefficients were obtained for both methods (>0.999). Results from both Kinetic Chromogenic LAL-based were within the method variability.
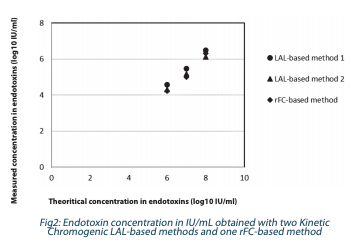
In Figure 2, results from the two LAL-based methods were compared to one rFC-based method.
The same results were obtained independently of the test method (within method variability) for high endotoxin concentrations. In this example, endotoxin testing was used to support process consistency. The recommendation was to use only one method to avoid any bias.
To conclude, here, we demonstrated that Kinetic Chromogenic LAL and rFC-based methods can be used to quantify accurately and reproducibly high endotoxin concentrations from a Gramnegative bacterium.
b. Example of analytical support to help the bioprocess teams in defining the most appropriate endotoxin removal steps
Below is an example of analytical support given by our lab to help the bioprocess teams in defining the most appropriate extraction and purification technologies for recombinant proteins produced on Gram-negative bacteria.
Endotoxin removal steps are critical to implementing but in general, there is no universal protocol that can be broadly applicable. The different characteristics of the production process and target protein or antigen require specific method combinations to achieve successful endotoxin removal, and the endotoxin testing method procedure must be adapted each time.
In our example, the evaluated endotoxin removal methods included a combination of purification technologies and treatment with extreme pH, precipitation using salts, and detergent used to dissociate the protein-endotoxin complex. Different ion strengths were also used to elute endotoxin.
In the first intention, the compendial Kinetic Chromogenic LAL method was selected to monitor endotoxin removal.
Our Kinetic Chromogenic LAL testing was limited in providing accurate detection in complex matrices, the downside of the purification treatments being strong interferences on the LAL enzymatic cascade. This was observed by low PPC spike recovery that was fixed by dilutions in endotoxin-free water, a common treatment used in the endotoxin community, to overcome product interferences.
By applying high dilutions, results obtained by the Kinetic Chromogenic LAL method were not satisfying since they were “signed” (e.g., <5000 IU/mL). Usually, the obtention of signed results is minimized by having a maximum valid dilution (MVD), which is derived from the ratio of the endotoxin limit to the test sensitivity. However, for intermediate stages, no product specification nor MVD are defined; high dilutions can be applied to recover PPC proper values.
Signed results did not indicate the efficiency of the tailored combination of purification technologies. Indeed, the use of extreme pH or detergent, for instance, helps endotoxin removal, but the drawback of those methods includes the need for subsequent detergent removal in samples, buffering, etc. especially in LAL based methods.
One solution would have been to increase PPC concentration. Indeed, the statistics for the recovery of our current PPC (0.5 IU/mL) may not be optimum. Another solution would have been to address the multiple sample interferences sequentially. For instance, an acidic pH (<6) and the presence of organic solvent could have been addressed with dilution in endotoxin-free NaOH to adjust the pH followed by multiple dilutions in endotoxin-free water.
The phage ligand-based assay looked very promising to overcome our sample interferences despite its longer testing time compared to classical LAL and rFC assays. This assay is based on a selective capture of lipopolysaccharide (LPS) achieved using a phage-derived receptor protein. After binding the sample’s LPS to the microplate, the original sample matrix is washed off, thereby eliminating potentially interfering components. In the second stage of the assay, LPS is detected by factor C that reacts with a fluorescence substrate (Grallert et al., 2011).
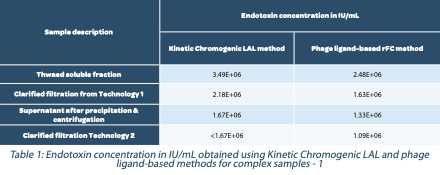
In Table 1, we first demonstrated that for non-interfering matrices, the same results were achieved between Kinetic Chromogenic LAL and phage ligand-based assay.
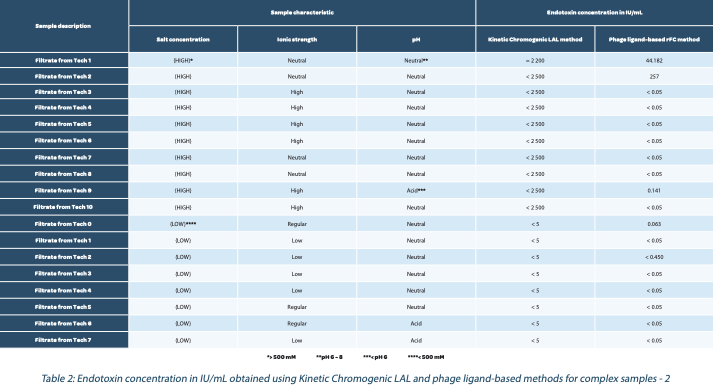
In Table 2, we implemented phage ligand-based assay to achieve better assay sensitivity. The additional washing steps allowed reduction of sample dilution to 1:1 or 1:10.
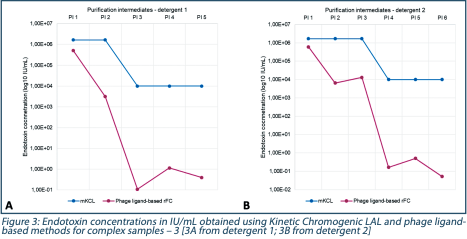
In Figure 3, purification intermediates were screened with both methods. In blue, results obtained from the LAL method, only signed results were obtained (i.e., < 1666616 and <10000 IU/mL) while in fuchsia quantified results were obtained (except for PI 5 detergent 2, signed result <0.5 obtained (Figure 3B)).
The performance of purification tools was assessed to the phage ligand-based assay since it was the only method able to show the decrease of endotoxin content over purification steps.
For this study, endotoxin levels needed to be closely monitored to define the efficiency of reduction or elimination steps through the different purification and manufacturing stages. The rFC-based method was the adapted solution.
The phase-ligand based assay allowed us to meet our bioprocess needs and was an excellent “orthogonal” tool.
To conclude, in our lab, the rFC method meets our needs in our settings for final product release, environmental water testing, and MPM/IPC testing.
Conclusion
The rFC based method is not widely approved as a compendial test, and most pharmacopeias are still considered it as an alternative method.
However, pharmacopoeias are in the process of evolution and propose draft and new texts on the recombinant approach of the LAL reagent. The Europeans are the first to have an official text that will be effective from January 2021, and the other pharmacopoeias should come with similar texts in 2022-2023 before finally being able to harmonize on this approach in about 5-6 years.
Partager l’article

Thierry Bonnevay – Sanofi
Thierry.Bonnevay@sanofi.com

Marine MARIUS – Sanofi
Marine.Marius@sanofi.com
Glossaire
BET: Bacterial Endotoxin Test
LAL: Limulus Amebocyte Lysate
rFC: recombinant Factor C
MPMT: manufacturing process monitoring testing
IPCT: in-process control testing
Ph. Eur.: European pharmacopeia
USP: United States pharmacopeia
JP: Japanese Pharmacopeia
ChP: Chinese Pharmacopeia
PDG: Pharmacopeial Discussion Group
PF: Pharmacopeial Forum
Références
1. Grallert, H., Leopoldseder, S., Schuett, M. et al. EndoLISA®: a novel and reliable method for endotoxin detection. Nat Methods 8, iii–v (2011).
2. Marius, M., Vacher, F. and Bonnevay, T. Comparison of LAL and recombinant Factor C endotoxin testing assays in human vaccines with complex matrices. PDA Journal of Pharmaceutical Science and Technology 10.5731/pdajpst.2019.010389 (2020)
3. Pharmacopeial Forum USP. Stimuli articles. Vol. 36(1). A Recombinant Factor C Procedure for the Detection of Gram-negative Bacterial Endotoxin. Bruce Loverock & al. (Jan.–Feb. 2010)
4. European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (Ph. Eur.) 10th Edition Chapter 2.6.14. Bacterial Endotoxins. Strasbourg, France: EDQM Publications (2016).
5. Chapter <85>, Bacterial Endotoxins Test. United States Pharmacopeia 39. Rockville, MD: U.S. Pharmacopeial Convention (2015)
6. Chapter <1225>, Validation of Compendial Procedures. United States Pharmacopeia 39. Rockville, MD: U.S. Pharmacopeial Convention (2017)
7. General Test 4.01 Bacterial Endotoxins Test. Japanese Pharmacopoeia (17th Edition). Japan: The Japanese Ministry of Health, Labour and Welfare; p. 110-3 (2016)
8. Kikuchi Y, Haishima Y, Fukui C, Murai T, Nakagawa Y, Ebisawa A, et al. Collaborative study on the bacterial endotoxins test using recombinant Factor C-based procedure for detection of lipopolysaccharides. PMDRS; 48:252-60 (2017)
9. U.S. Food & Drug Administration Guidance for industry: pyrogen and endotoxins testing: questions and answers (2012).
10. United States Pharmacopeial, In-Process Revision: <1085> Guidelines on the endotoxins test. Pharmacopeial Forum Online, 4 (2018)
11. European Directorate for the Quality of Medicines & HealthCare, European Pharmacopoeia 10th Edition: Chapter 5.1.10. Guidelines for using the test for bacterial endotoxins. EDQM Publications: Strasbourg, France (2016) 12. European Pharmacopeia, Pharmeuropa 31.1 , New chapter 2.6.32 “Test for Bacterial Endotoxins using recombinant factor C” , published in Ph. Eur. Version 10.2 and applicable in version 10.3 (January 2021)
13. PMDA, Japan. Publication new draft for comments “Bacterial Endotoxins Test and alternative methods using recombinant protein-reagents for endotoxin assay” (October 2019)
14. United states Pharmacopeia, Pharmacopeial Forum 46 (3). New Stimuli to the revision process “Application of Recombinant Factor C Reagent for the Detection of Bacterial Endotoxins in Pharmaceutical Products and Comparability to Limulus Amebocyte Lysate”, Jay Bolden (2020)