Sommaire
- Comment accélérer la libération grâce aux CAPA ?
- La libération pilotée et maîtrisée par le management Visuel
- Dossier de Lot Electronique : les opportunités et les pièges à éviter
- Le défi de la digitalisation des enregistrements de production
- AQU@Sense MB: A technological evaluation for « on-line » pharmaceutical water analysis
- Individual closed isolators for cell therapy
- Evaluation of a Quaternary Ammonium Ready To Use (RTU) Disinfectant and Hydrogen Peroxide/Peracetic Ready To Use (RTU) Combination Sanitization Regimen for Cleanroom Start-Up
- Introduction à la norme ASTM E3263-20 "Standard Practice For Qualification Of Visual Inspection of Pharmaceutical Manufacturing Equipment And Medical Devices For Residues" (Standard pour la qualification de l'inspection visuelle des équipements de fabrication pharmaceutique et des dispositifs médicaux pour les résidus)
Individual closed isolators for cell therapy
Cell therapy (CT) is the paragon of what anyone could expect in case of cell disorder with individual blood collection, cells separation and cells modification before inject to the patient the repaired cells. The details of the working process to succeed have been describedbyIS E(1).Recent advances with CAR-T and CRIS R have helped in the fine separation technology between the good and the bad parts of the cells.
The environment in which these handlings have to be done must follow the current Good Manufacturing Practices (cGMP) meaning that beyond the research and development there must be logic of producer to implement an installation fitting with CT specific regulations. (2-3).
There are 2 choices of environment to isolate an aseptic bio-pharmaceutical process from its immediate surroundings either A in B or isolator in C or D. These A, B, C and D classes mean not only a defined ventilation/filtration, a speed of air and a filtered air renewal but a specific type of gowning for the personnel with a time for gowning. The choice of isolator with a class C or D surroundings according to local regulation facilitates the training of the operators and eases the process. (4).
Isolator for Cell Therapy with integrated lock chamber for transfer of components, incubator and centrifuge has been presented to the market in 2016 (5). This type of equipment fits perfectly for the research and development of sequences to be then applied in daily routine.
In case of multiple CT process each individual cell selection has to be separated and autonomous.
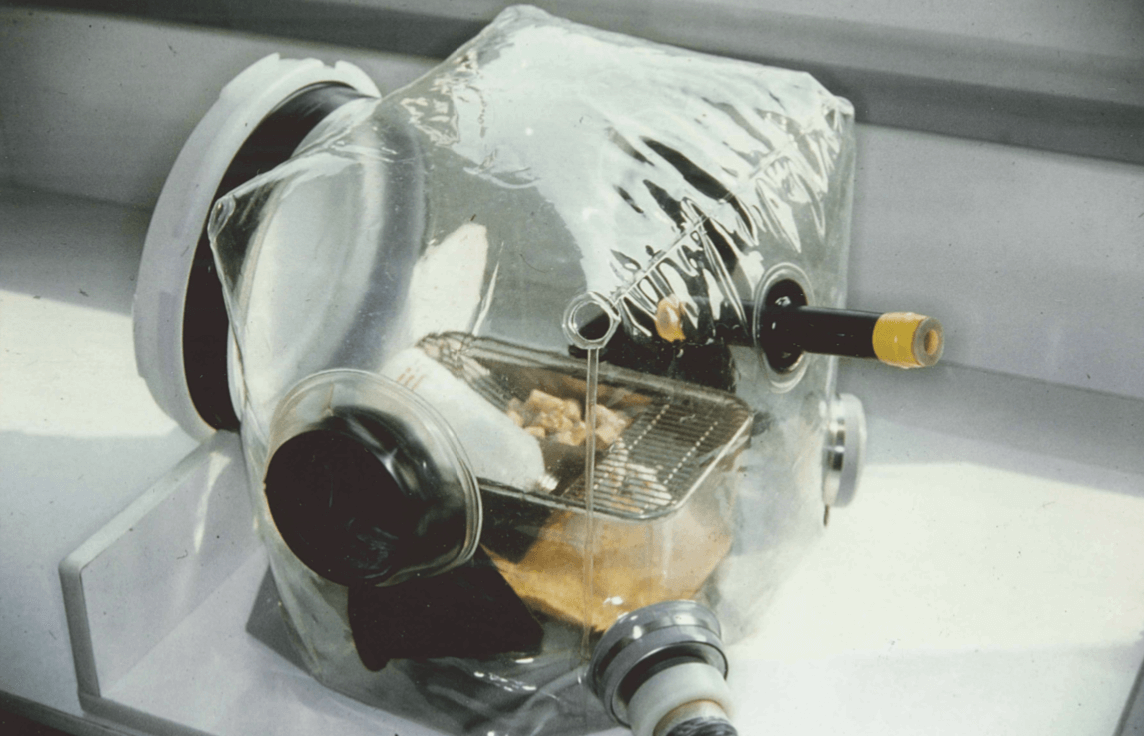
A solution is the use of multiple individual isolators, which is a variation of what has been done with success at INRA (French National Institute for Agronomical Research) for the study of intestinal flora in various types of monoxenic mice (Photo1) using flexible sterile closed isolators. The one proposed actually for CT is Gamma sterilized single use and ready to use.
Flexible film closed isolators have been described and used from the late 40s in sterile surgery (6) and from the late 50s in breeding of lab germ-free rodents (7).
A recent application with flexible disposable sterile isolator for the fill & finish of aseptic solution has been described (8).
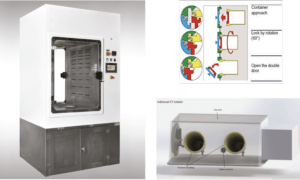
The set-up here of the cleanroom is a ballroom concept. All items and materials to be introduced in the cleanroom are bar coded or equivalent, sterile and double wrapped before to go through a H2O2 lock chamber (Photo 2) after removing the first wrapping.
The cells coming from the outside could be frozen at minus 70 °C. Their entrance in the cleanroom is done through an Alpha/Beta RTP transfer system (Figure 1) which fits with the Beta entrance door of each individual CT isolator (Figure 2).
The CT isolator is a transparent flexible positive pressure disposable sterile ready to use mini- isolator of approximate150 liters volume with adjacent forearms handling, top view and a 190 RTP Beta door to be connected alternatively to the Alpha door for the entrance of the cells, to the storage isolator and to the service isolator for the loading of the required items allowing the operator to proceed. The ventilation/ filtration system of each of these isolators is composed of an inlet 0,5 micron hydrophobic membrane filter and an outlet HEPA filter. The gas used to inflate the isolator can be either regular compressed air or any combination of compressed gas to increase the development of the cells (with added CO2 for instance). The positive pressure is in the range of 30 to 50 Pa. This mini-isolator includes a “tail” as a sleeve for external thawing.
Before to be used the airtightness of each of the individual isolator is tested according to ISO 10648-2 as its inlet membrane filter is integrity tested by a water intrusion test or equivalent. Each isolator is Gamma sterilized as other types of single use bags with Beta RTP connection (11).
Key point in this configuration is the use of Alpha/Beta RTP systems for various loading and unloading without any risk of cross contamination from viable and non-viable particulates. This type of DPTE® like connection with a rotation is QC/QA by its supplier for the quality of the gaskets and the safety during multiple connections (9-10)
Main point about this very installation is the only use of “closed isolator systems” as described in (4a): “Excluded external contamination from the isolator’s interior by transfer material via aseptic connections to auxiliary equipment rather than using openings to the surrounding environment. Closed systems remain sealed throughout operations” which is a warranty against any cross contamination with the surroundings.
Other important points to consider being in accordance with the User Requirement Specifications (URS) of most of the cell therapy process are:
• Cells transfer
• Thawing
• Centrifugation • Incubation
• Waste
The cells are transferred directly to their isolator from outside of the cleanroom through the RTP system. In the case of frozen cells at minus 70°C the isolator includes a sleeve to be dipped in an external WFI water bath or equivalent during the necessary time to thaw.
Ready to Use sterile RTP containers are used as centrifuge rotors allowing off the shelf refrigerated or not centrifuge to be used without any integration and modification.
Incubation according to the flow of work can be done in an incubation room at 35°C as a part of the cleanroom or in a specific RTP container which have to be placed in an appropriate incubator or in the incubator of the service isolator.
The waste is biologically inactivated in a two-door autoclave or equivalent before to be incinerated outside.
At the end of the process the modified cells exit the cleanroom by a proper pass-through.
The whole system is under a Data Integrity (DI) umbrella including the storage and the process with defined protocols and component lists to control the circulation of cells and components from a to z to know exactly at what stage are each of the cells process.
Both the storage isolator and the service isolator have a Unidirectional Flow (UDF) ventilation/HEPA filtration to sweep away the fastest possible any trace of H2O2 during the aeration post bio- decontamination phase. The accepted value of the H2O2 left level is part of the validation of the process.
The storage isolator (Picture 3) keeps sterile the necessary components and the service isolator (Picture 4) allows the transfers to the targeted isolator through its vertical (centrifuge bowls) and horizontal (components) RTPs. A tentative of a turnkey set-up is sketched in Figure 3.

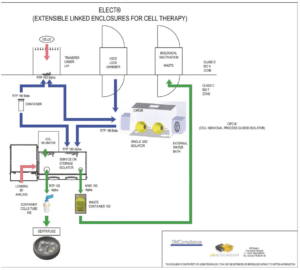
Thanks to this very design, the use of individual single use stringent sterile closed isolators first avoids cleaning and cleaning validation and when combined with a full decentralized process it provides for CT daily routine a “Never Touch” chain within a Class C comfort for the personnel and offers a maximum product/personnel cross protection. The added Capex for the storage and the service isolators to the whole investment is compensated by a low Opex (individual isolator/centrifuge rotor). The energy and compressed gas consumptions are minimized in accordance with the small volumes of the isolators.
Partager l’article

Didier Meyer – DMCompliance
Didier Meyer has spent most of his career in separation technology and isolation technology on the supplier side. He is currently consultant at DMCompliance and member of the A3P Board.
dgastonmeyer@gmail.com
Acronyms
CT Cell Therapy
ISPE International Society of Pharmaceutical Engineers
CAR-T Chimeric Antigen Receptor for T lymphocyte cells
CRISPR Clustered Regulatory Interspaced Short Palindromic Repeats
cGMP current Good Manufacturing Practices
INRA Institut National de la Recherche Agronomique
RTP Rapid Transfer Port
HEPA High Efficiency Particulate Air (filter)
DPTE Double Porte de Transfert Etanche
UDF Unidirectional Airflow
URS User Requirement Specifications
WFI Water For Injection
References
(1) https://ispe.org/pharmaceutical-engineering/november-december-2020/flexible-facility- design-multiple-cell-therapy
(2) US Food and Drug Administration. “Approved Cellular and Gene Therapy Products.” 29 March 2019. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/ approved-cellular-and-gene-therapy-products
(3) European Medicines Agency. “Advanced Therapy Medicinal Products: Overview.” 23 July 2020. https://www.ema.europa.eu/en/humanregulatory/overview/advanced-therapy- medicinal-products-overview
(4) a)Points to Consider for the Aseptic Processing of Sterile Pharmaceutical Products in Isolators 2020 Parenteral Drug Association, Inc.
b)US Food and Drug Administration. Guidance for Industry Sterile Drug Products
Produced by Aseptic Processing – Current Good Manufacturing Practice, US Department of Health and Human Services. Rockville, Md., 2004
c)Eudralex , The Rules Governing Medicinal Products in the European Union: Volume 4, EU Guidelines to Good Manufacturing Practices for Medicinal Products for Human and Veterinary Use-Annex 1, Manufacture of Sterile Medicinal Products; European Commission 2008. http://ec.europa.eu/health/files/eudralex/vol-4/2008_11_25_gmp-an1_en.pdf(accessed November 19, 2014)
(5) SANYO Integrated Cell Processing Work Station CPWS PPLF-4205
(6) United States Patent Office Nr 2473033 Sterilized and Air Conditioned Chamber for Surgical Uses
(7) A history of isolator and containment technology Part 1: Early containment leading to flexible film isolators by Doug Thorogood Clean Air and Containment Review | Issue 18 | April 2014
(8) The partner of choice for the formulation, development and GMP manufacturing of nanomedicines completed with aseptic filling. Pharma Post N°4 Digital Supplement October 2020 from page 52. https://fr.zone secure.net/167165/1247799/#page=52V-nano
(9) The use of enhanced GMP manufacturing techniques boots the success of large scale production of EMA approved cartilage substitute by Giulietta Roel of Codon AG and Marco Fadda of Comecer at A3P Barrier Technology Days 19/20th of March 2019 Pau (F)
(10) MW SAFECAN JCE MultiWay System® 190 Rigid container
(11) Minimizing Contamination Risk In SU Final Filtration & Filling By Mehdi Boukami. Merck KGaA Field Marketing Single-Use Technology –EMEA September 17th 2020, Molsheim France