Sommaire
- Comment accélérer la libération grâce aux CAPA ?
- La libération pilotée et maîtrisée par le management Visuel
- Dossier de Lot Electronique : les opportunités et les pièges à éviter
- Le défi de la digitalisation des enregistrements de production
- AQU@Sense MB: A technological evaluation for « on-line » pharmaceutical water analysis
- Individual closed isolators for cell therapy
- Evaluation of a Quaternary Ammonium Ready To Use (RTU) Disinfectant and Hydrogen Peroxide/Peracetic Ready To Use (RTU) Combination Sanitization Regimen for Cleanroom Start-Up
- Introduction à la norme ASTM E3263-20 "Standard Practice For Qualification Of Visual Inspection of Pharmaceutical Manufacturing Equipment And Medical Devices For Residues" (Standard pour la qualification de l'inspection visuelle des équipements de fabrication pharmaceutique et des dispositifs médicaux pour les résidus)
AQU@Sense MB: A technological evaluation for « on-line » pharmaceutical water analysis
THE «BIOBURDEN» CONTROL is a release test which allows the microbial load of products to be determined. In accordance with the standards of the Pharmacopoeias, it can be carried out via a culture step on a specified medium (R2A plates for water testing) by filtration, surface spreading or by in-depth seeding. The result obtained after 5 regulatory days of incubation at 30-35°C is expressed in CFU (Colony Forming Unit) per volume filtered.

This Pharmacopeia test has known limitations, such as the long duration before obtaining the result, the high rate of handling errors or a poor recovery of the microflora (<1% cultivable).
Alternative methods were first developed to reduce analysis time and are already in place in the laboratory performing the tests. On the industrial side, real-time analyzers are now required in order to monitor the “bioburden” and manage contamination control.
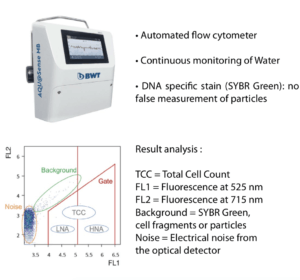
New technologies are now applied to the “monitoring” of water systems: AQU@Sense MB is marketed by the company BWT, the principle of microbial detection is based on DNA labeling and analysis of fluorescence events by flow cytometry.
Water is a key element in the pharmaceutical industry because it is used as a raw material, diluent, or even as a final product. There are 2 grades of pharmaceutical water: Purified Water (PW) and Water.
For Injection (WFI). The microbiological control of water is thus strictly regulated:
The AQU@Sense MB counts the actual bacterial cells present in water, accurately & within 20 minutes. Even bacteria which are in a stress-induced state, that cannot be cultivated (called VBNC), are detected by this flow cytometer. Additionally, the method does not depend on incubational and nutritional requirements.
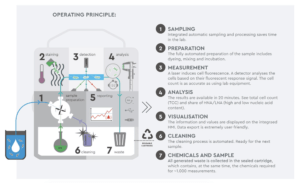
The objective of the technological evaluation of AQU@Sense MB is to determine the performance of the system applied for on-line water monitoring 1) by generating data from laboratory assays using known and calibrated microbial strains and 2) by performing measurements and testing different water loops at sample points.
• Equipment installation and training of personnel < 1 day
• Protocol for sampling, testing and analysis according to BWT procedure (see operating principle) < 30 min
Study Design Space = Lab assays (microbial testing) and on-line water monitoring of different water loops (production or distribution)
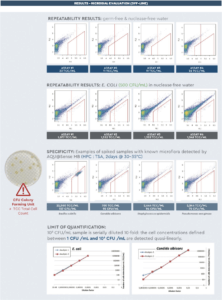
• Different matrix assessed (D fl uid, TBS buffer & nuclease free water) = compatibility verifi cation (no matrix interference)
• Spiking assay with known and calibrated strains (ATCC reference Pharmacopeia strains (500 CFU bioball – bioMérieux) + 2 wild isolates (from Marcy L’étoile, < – 70°C frozen samples)
- specificity < 100 CFU = detection of all viable microflora
- range & linearity (between 10 to 106 CFU – duplicate assays) • Repeatability assay (4 replicates)
• Monitoring of PW loops (production & distribution installation of PW) and « bioburden » testing according to the current method
« Bioburden » testing is performed according to the Pharmacopeia method by a qualified person: 100 mL sample is filtered (duplicated assay) using Milliflex cassettes (R2A Agar medium) that are incubated at 30–35°C during at least 5 days. Results are interpreted at the 6th day of incubation by observation of colonies present on the surface of the membrane: number of CFU (Colony Form Unit) per filtered volume is then determined.
Inoculum control (duplicated assay) is performed by culture on Agar plates (TSA) incubated during 2 days at 30–35°C.
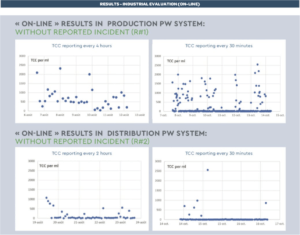
The TCC data collected continuously showed the importance of the analysis frequencies. Online FCM enables a detailed characterization of the frequency and magnitude of microbiological fluctuations on a level of detail not possible with standard methods.
« ON-LINE » RESULTS IN PRODUCTION PW SYSTEM: with 2 series of reported incidents (R#2) A TCC baseline is present with some sporadic events that do not directly correlate with variations in the physical parameters of the loop (fl ow, filling of the tank, pressure, total organic carbon, or conductivity), expect for a reported chlorometer issue with abnormal injected volumes of NaOH (Oct. 30th and Nov. 3rd). The associated events clearly indicate a microbiological problem (see examples on the right).
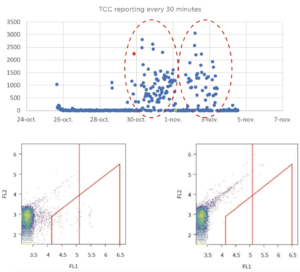
As a first example, the reported 2,240 TCC (red point) is clearly associated with a microbial event (as indicated by the bacterial clusters within the gate). These cells are possibly VBNC, as the plate count showed no elevated number of CFU.
As a second example, no microbial event is reported = 0 TCC (green point part of the baseline).
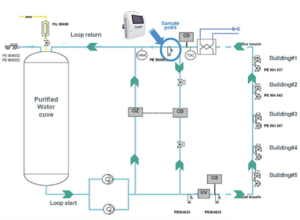
ON-LINE MEASUREMENTS WERE PERFORMED DURING DIFFERENT PERIODS OF TIME AND AT DIFFERENT SAMPLE POINTS FROM PW LOOPS OF PRODUCTION OR DISTRIBUTION SYSTEMS:
• R#1 system produces a demineralized and osmosis treated PW characterized by 0.04 μS/cm conductivity, 3 ppb TOC with a variable fl ow: 40 – 60 m3/hr.
• R#2 system produces EDI and osmosis treated PW characterized by 0.06 μS/cm conductivity, TOC=1 ppb with a variable fl ow: 9 – 13 m3/hr. (EDI: Electrodesionization)
• R#2 distribution system is illustrated above.
Conclusion
The microbial results have allowed to check the performance of the AQU@Sense MB in terms of microbial detection in high concentration ranges (from 1 to 106 CFU/mL), except for mold (Aspergillus brasiliensis).
The technological principle remains simple and the device implementation is fast. Alert and action limits are still to be defi ned during “on-line» monitoring. Microbial quality of Purifi ed Water is by this technology continuously monitored and controlled to prevent contamination.
This initial industrial assessment has been performed using the fi rst generation of the cartridge (TCC). BWT has recently introduced a new cartridge with an improved chemical formulation, which can directly quantify the number of viable bacteria based on the assessment of bacterial membrane integrity (ICC: Intact Cell Count), and it should be tested further!