Sommaire
- Overview of communications from health authorities on remote inspections and audits
- Hesitant beginnings with a sustainable method.Experience feedback
- Remote audit. The role of the Covid crisis in its generalization, principles, technologies and auditor profiles
- The remote audit: strengths & weaknesses
- Microbiological study on the management of holes in gloves for isolators
- Contamination Control Strategy: practices & a case study of a CCS implementation
- Toxicological approach to define the PDE for your cleaning validation process
- How high performance materials for secondary packaging components can significantly reduce particle contamination in Ready-to-use vials
- Etes-vous prêts à WUSUTer ?
Finding the perfect match. How high performance materials for secondary packaging components can significantly reduce particle contamination in Ready-to-use vials
The global pharmaceutical is going significant with the advent of small batch personalized medicines. The drug pipeline is filled with more than 4500 injectable drugs in various phases, 80% of which are biologics (Global Data). It is a formidable challenge to bring these drugs to market fast while keeping costs under control. All stakeholders in this industry have the inherent goal to make injectable drugs safe and effective.
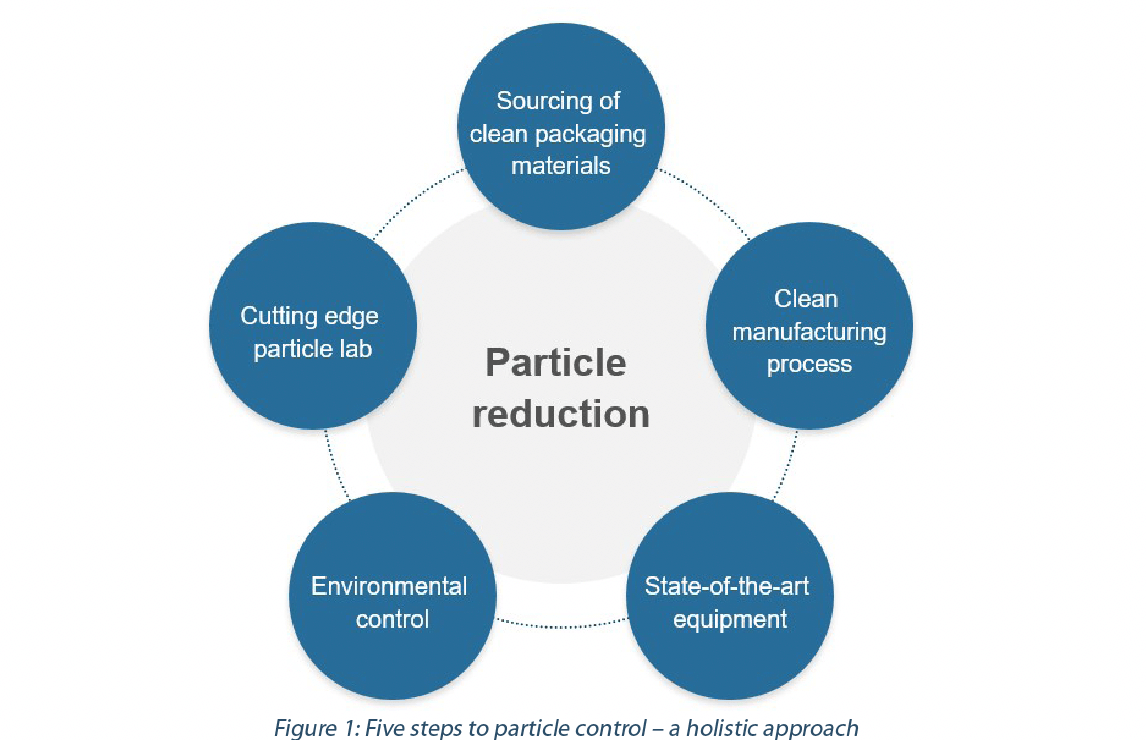
To address these challenges, the pharma industry is turning their focus to Ready-to-use (RTU) containers. This is manifested through a number of trends. Emerging biopharma companies have started to retain greater control of their assets by launching and filling an increasing number of drugs themselves. At the same time the share of drug filling operations being outsourced to C(D)MOs keep increasing, with larger C(D)MOs embracing smaller clinical trial fills and installing flexible filling equipment that is geared towards RTU containers(1). This strategy is also being implemented by big pharma, as batch sizes continue to dwindle and flexibility in fill-and-finish is key. However, caution is warranted when choosing RTU containers, as the secondary packaging for these presentations (tub, nest, header bags, and materials known under the brand name DuPontTM Tyvek®, which are hereinafter referred to as sealing or lid material) are an additional, potential, source of foreign contamination. While the nested presentation greatly reduces the risk of damage to the containers, contamination coming from the introduction of additional materials needs to be well understood and controlled. In order to do this, a layered, holistic approach (Figure 1) to contamination control is required. (see Figure1)
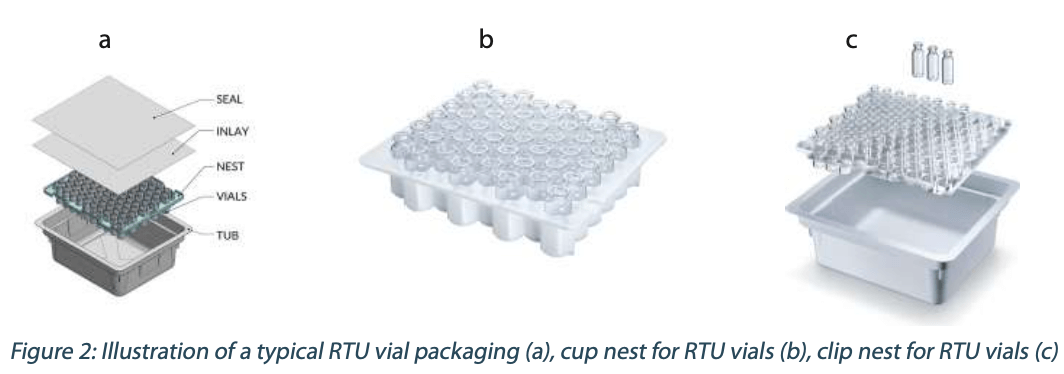
In this paper, we discuss one of the key RTU packaging components, the so-called “nest” (Figure 2). Nest designs may vary depending on the container type – vial, prefillable syringe or cartridge – but they have a common purpose. To keep the containers in place during storage, transportation and handling, to avoid glass-to-glass contact, and present the container precisely to the filling needle. The nest is a critical component, therefore a careful selection of the material, design, production, and control processes is needed to ensure the quality of the RTU containers.
Our discussion in this paper will focus on the material selection for the nest and how a systematic approach can help to improve the quality of RTU vials significantly.
1. Quality of RTU vials
The final quality of RTU vials directly depends on the material selection and design of the primary and secondary packaging. A poorly designed nest can impact the quality of the vial in any of four ways:
1) physical damage to the vials themselves (breakage, checks, scratches)
2) increased levels of bioburden/endotoxin
3) internal and/or external particulate
contamination (foreign contamination, vial marks)
4) inaccurate presentation of the vial to the
feeling needle (pitch, angle, alignment, center).
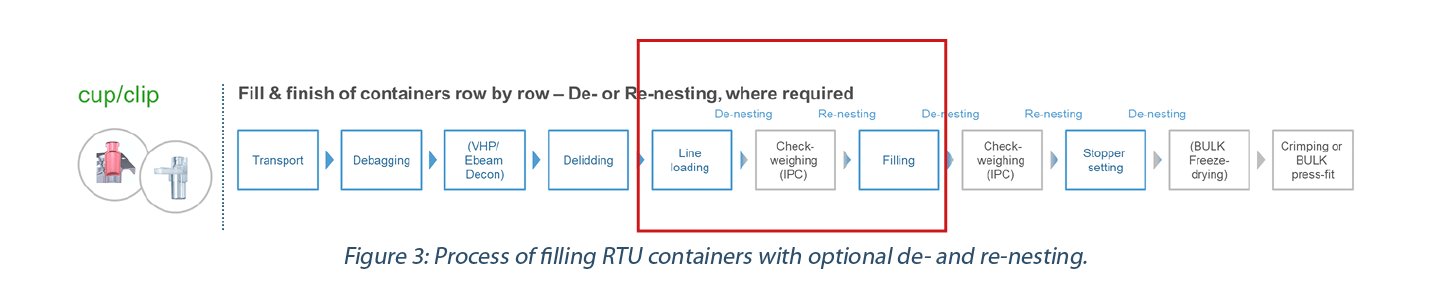
With the ready-to-use option, new components are introduced such as the nest and sealing material, which are in direct contact with the vial during transportation, storage and handling. Packaging components must maintain product quality throughout sterilization, transportation, and filling process integration (Figure 3). While some machines allow the containers to remain nested for all fill-and-finish steps, customers often choose to remove (de- nest) the containers for certain process steps such as check-weighing at full line speed or application of the closure and place them back into the nest (re-nesting) as needed. The next abstract will explain why special attention should be given when removing the containers from their “safe” place inside the nest.
2. Problem statement
Friction on vial insertion/removal and vibration during transportation and handling resulting in mechanical abrasion and shed nest material must be considered as a potential source of particles in/on RTU vials. It is essential to reduce the friction between the glass and the nest.
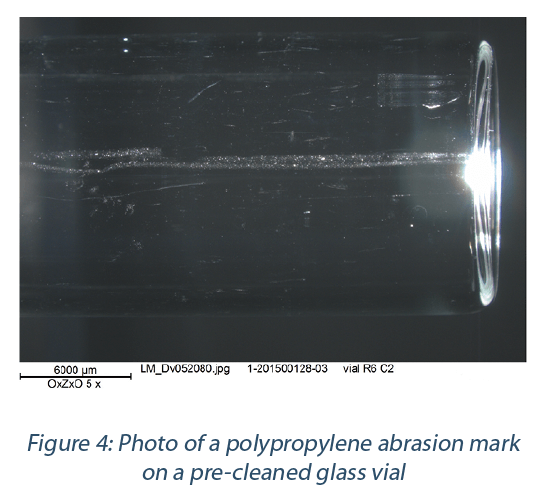
Issues of abrasion of polymers leaving sub- visible particle aggregates (debris fields) on the glass have been documented with freshly washed glass vials after a single placement- removal cycle in a polypropylene nest. These defects are localized in the areas where the polymer material is in direct contact and moving relative to the glass surface (Figure 4.). The abraded particles were analyzed by SEM- EDS as primarily carbon and identified by FTIR-ATR as Polypropylene. This observation supports that a polypropylene nest can lead to mechanical abrasion and external, sub- visible particle aggregates resulting from the glass-nest interface.
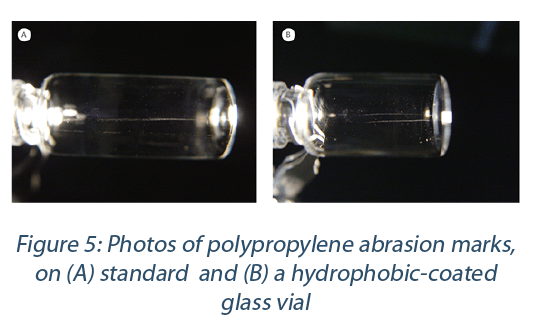
One option to reduce the risk of abraded particles, is to smoothen the glass surface. Tests on different glass surface treatments have been performed, like plasma and CO2 cleaning, hydrophobic plasma coating, and acetylene treatment. These tests were performed with un-cleaned and cleaned vials[2].
The surface in the test with lowest friction were vials with a hydrophobic outer surface coating (Figure 5). The examples in Figure 5 demonstrate that the investigation of surface friction reduction on the glass vial did not lead to significantly less visible abrasion of polypropylene or polypropylene artifacts on the glass. The conclusion of this investigation is that even the smoothest glass surface will result in mechanical abrasion of polypropylene and that polypropylene does not pose significant enough abrasion resistance for a direct polymer-glass contact.
Since the glass surface cannot be modified sufficiently to reduce mechanical abrasion, the nest material was examined in more detail to find a resin with the highest mechanical abrasion resistance, thereby reducing the potential for particles in RTU vial presentations.
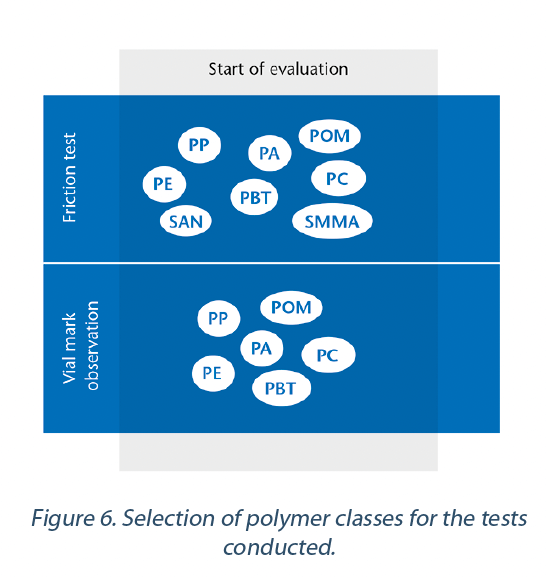
For the study, fifteen different samples were evaluated in eight different polymer classes (Figure 6) and four low friction coatings on nests. All these materials were tested for coefficient of friction and also visually for abrasion to identify the material with lowest abrasion potential.
3. Experimental methods and materials
Samples
All samples are technical products. They were purchased directly from the polymer material supplier or provided by the mold manufacturer.
Visual inspection of glass vials
The vials were visually inspected for abrasion marks after setting them into and rejecting them from the nest. This investigation was done based on EP 2.9.20 in an inspection chamber. For better visualization and documentation of the detected defects, additional light sources were used to get a better contrast in the photos.
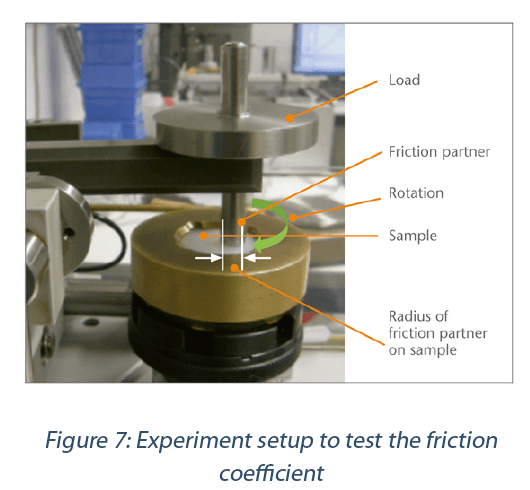
Tribometer (Figure 7)
The friction coefficient experiments were conducted with a tribometer based on model testing systems “Ball / disc, disc rotates” based on ISO 7148-2. The sample rotates under the friction partner (sapphire ball, packaging material). Depending on the friction value of the sample surface, a lateral force (friction force) arises which is measured.
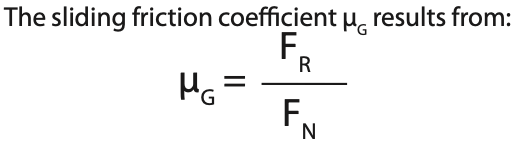
μG = coefficient of sliding friction,
FR = frictional force
FN = normal force
Two rotation lines, the inside curve (R4) and the outer curve (R6), were evaluated. The given results of the friction coefficient are the average values of both test results.
Only the first rotation of each curve was used to calculate the friction coefficient, since the surface may have changed after that. The damage to the polymer surface was evaluated at the end of the test.
Classification of the damages on the polymer plates was done visually by comparing and ranking the material deformation under halogen light against each other. The damages were documented by light microscopy.
4. Results and discussion
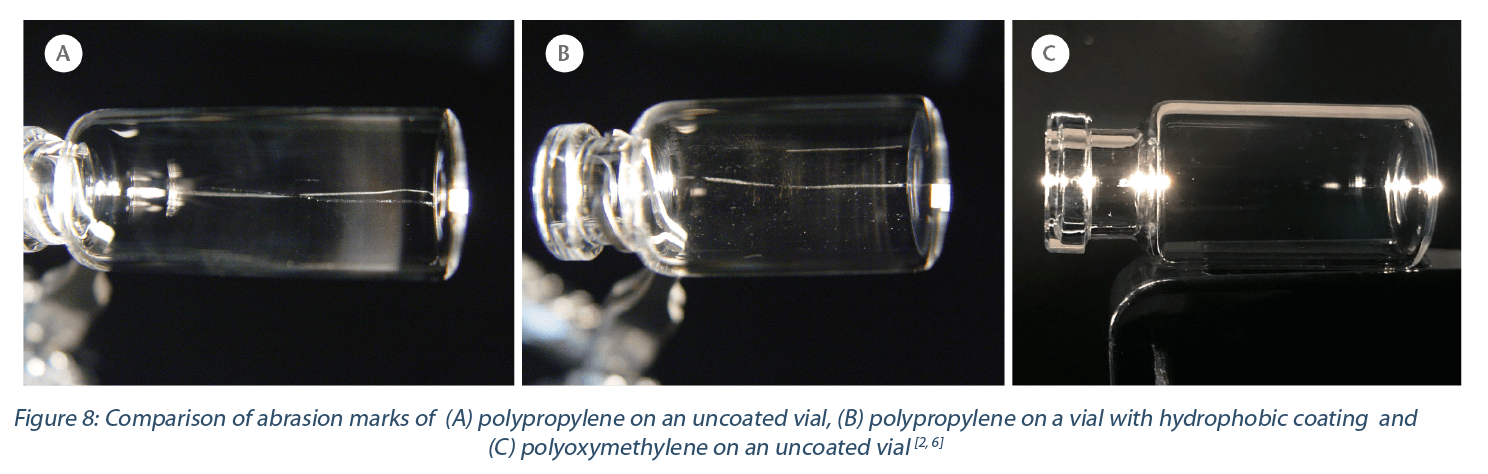
In the first set of experiments, six polymer materials, which were available in the nest design, were evaluated for abrasion marks on the glass surface after insertion and removal[4] (Figure 8). The intensity of the vial marking is equated to the likeliness of polymer abrasion. The best performance with none to nearly no visible marks was polyoxymethylene (POM) and polyethylene (PE), followed by polybutylene terephthalate (PBT), polyamide (PA), polycarbonate (PC) and the worst performing nest material of all – polypropylene (PP) and coated PP-material.
For the following experiments additional polymer materials were added to the tests. Polymers, which were not available in nest design, needed a different type of investigation. Therefore, a tribometer was selected for broad material screening of several pharma industry approved polymers (USP class VI compliant).
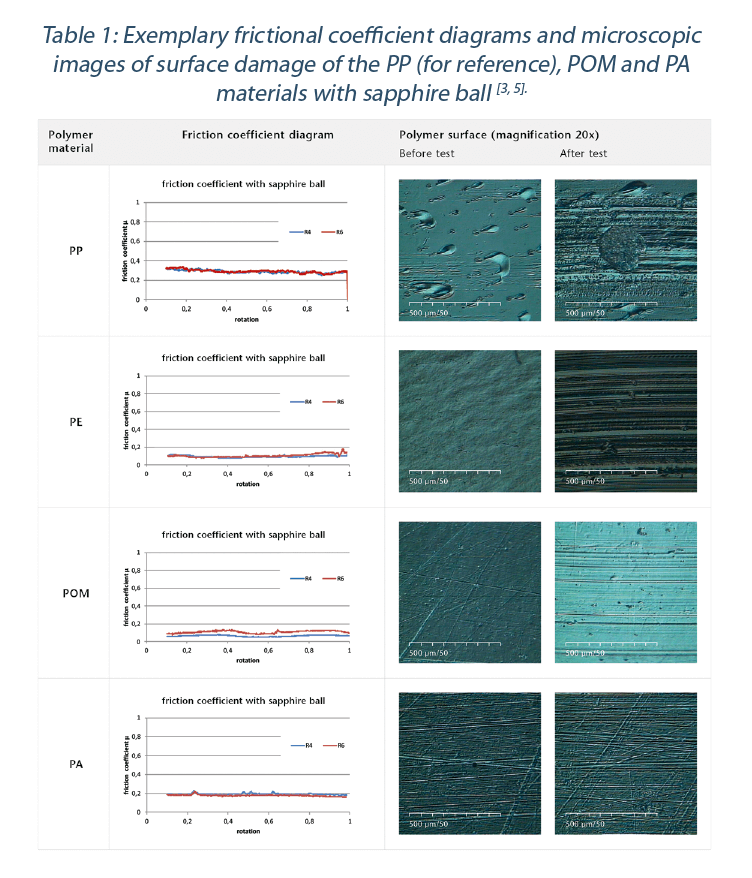
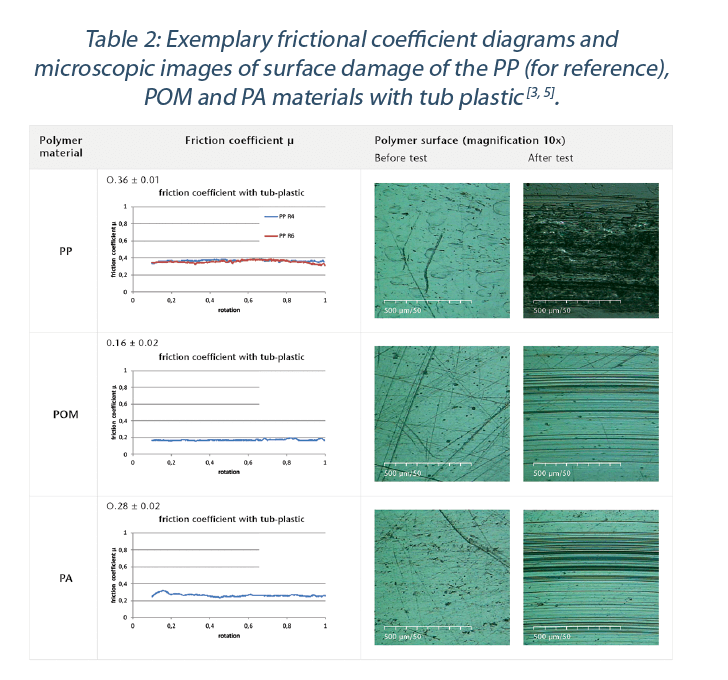
The test set up provides results on friction coefficient to a sapphire ball to simulate glass – polymer interaction. An important output is the characterization of the polymer material after the test, as the observed damages on the polymer material indicates directly the abrasion resistance and hence the risk of particle formation [3].
The observed friction coefficient and classification of visual damages of all tested samples are given in Table 1 and Table 2. POM, PA and SAN have the lowest friction coefficient values to the glass ball (Figure 9). Depending on the surface structure and the grade of the polymer, slight deviations between the polymer grades were measured[3,5]. The highest observed friction values were found for test samples made out of PP and PC. While PE had a low friction coefficient, it showed significant visual damages on the polymer surface (Table 1). The investigation of the polymer surface on damages reveals that the POM, PA and SAN materials have the highest abrasion resistance of all materials tested.
5. Conclusion
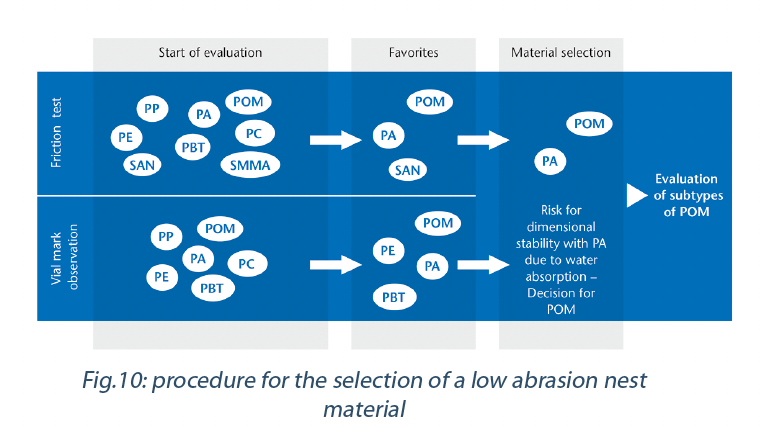
For the evaluation of the most appropriate nest material with a high abrasion resistance, a four-step process has been performed (Figure 10).
To summarize, the material investigation identified three materials (POM, PA, SAN) with low coefficient of friction combined with high to very high mechanical abrasion resistance i.e. minimal surface damage. Four materials (POM, PE, PBT and PA) were determined to generate low to very low visible artifacts i.e. material transfer of polymer to glass. As a result, the most suitable materials found were POM and PA. As PA is a polymer prone to dimensional control issues in humid environments, POM has been determined to be the most appropriate material for a vial nest for pharmaceutical applications.
This result should come as no surprise to pharmaceutical manufacturers, as POM is now commonly used for surfaces that come in direct contact with the glass containers, such as guide rails, scrolls and star wheels. This material has been proven to reduce glass damage and vial marks on bulk filling lines. Thus, it only makes sense to use such premium material to manufacture nests for high quality glass containers that will eventually store live saving drugs.
Partager l’article
John BERGGREN, DR. Inka HENZE & DR. Robert LINDNER – SCHOTT
Références
- [1] https://www.contractpharma.com/issues/2021-11-01/view_columns/pharmaceutical-packaging-trends-252591
- [2] Test ID. No. 1-2015-00128, SCHOTT AG accredited laboratories, date of report release 18. June 2015
- [3] Test ID. No. 5-2015-00125, SCHOTT AG accredited laboratories, date of report release 03. June 2015
- [4] Test ID. No. 1-2015-00298, SCHOTT AG accredited laboratories, date of report release 18. June 2015
- [5] Test ID. No. 5-2015-00218, SCHOTT AG accredited laboratories, date of report release 17. August 2015
- [6] Test ID. No. 5-2019-00379, SCHOTT AG accredited laboratories, date of report release 23. April 2020


