Sommaire
- Remot Audit. Point sur les communications des Autorités de santé sur les inspections et audits à distance
- Remote audit. Des débuts hésitants à une méthode pérenne. Retours d'expérience
- Remote audit. Généralisation, principes, technologies et profils d’auditeurs
- Remote audit : forces & faiblesses.
- Microbiological study on the management of holes in gloves for isolators
- Contamination Control Strategy: practices & a case study of a CCS implementation
- Toxicological approach to define the PDE for your cleaning validation process
- How high performance materials for secondary packaging components can significantly reduce particle contamination in Ready-to-use vials
- Etes-vous prêts à WUSUTer ?
Toxicological approach to define the PDE for your cleaning validation process.
HBELs (Health-Based Exposure Limits) is a general term described in EMA/CHMP/ CVMP/ SWP/169430/2012 guideline.
It gathers different approaches that enable, after safety evaluation, to define a level of acceptable daily dose for a specific substance to which a target organism (human/ animals) will be exposed:
– In Industrial Hygiene, the HBEL corresponds to an OEL (Occupational Exposure Limit) expressed in mg/m3 of surrounding air inhaled by an operator in 8 hours (or OEB: Occupational Exposure Band if limited data set is available) ;
– In Pharmaceutical Quality (topic discussed in this article), the HBEL is used to conduct the risk evaluation related to potential cross-contamination in manufacturing of pharmaceutical products intended for human/animal patients. The HBEL corresponds to a PDE (Permitted Daily Exposure) expressed in mg/kg bw/day of solvents or residual substance that can be either an API (Active Pharmaceutical Ingredient) or an inactive ingredient (excipient).
In the field of chemicals, agrochemicals or food industry, other acronyms also address the HBEL notion: TDI (Tolerable Daily Intake), ADI (Acceptable Daily Intake), or ADE (Acceptable Daily Exposure). In the pharmaceutical industry, the PDE value, specific to each substance of concern, is used by the cleaning validation specialists to establish the MACO (Maximum Allowable Carry Over) value, to demonstrate that cleaning operations are well adapted and to assist with decisions regarding the use of dedicated equipment or facilities for high potent APIs.
Historically, PDE used in the cleaning validation processes were determined based on LD50 (Lethal Dose 50%) or based on the therapeutic dose weighted by a safety factor (1000). These approaches could lead to overestimate or under-estimate the risk for each substance.
Since 2012, EMA developed a specific guideline to define a more accurate and comprehensive toxicological approach for the determination of PDEs (EMA 2012; EMA Q&A 2018). A similar approach for PDE determination is addressed in VICH GL18 (R) related to residual solvents in veterinary medicinal products.
1. The PDE monograph
The recommended method of PDE determination considers all the pre- clinical and clinical available safety data for the substance of concern. The route of administration (oral, parenteral, dermal, inhalation or any other specific route) and the target population (adults or paediatrics, any other susceptible subjects, or veterinary species) should be related to the exposure conditions of the residue in the next manufacturing run.
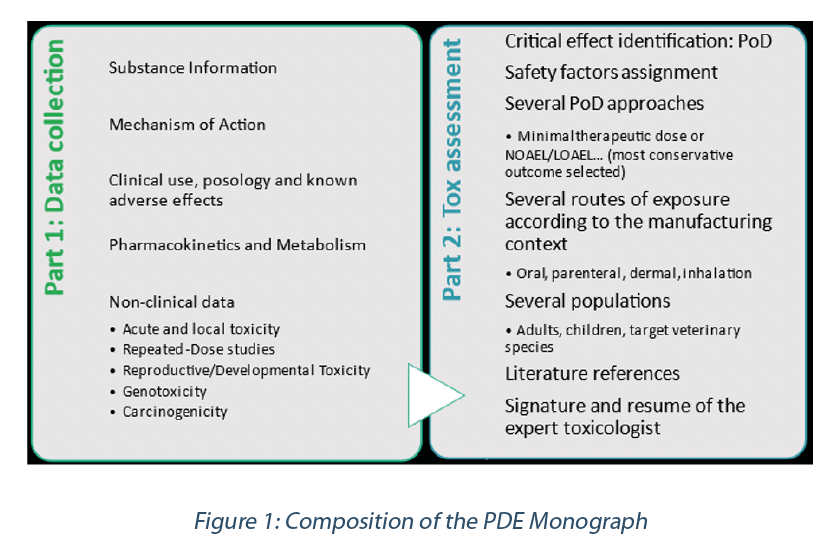
To fulfil EMA recommendations, the PDE Monograph includes two parts (as described in Figure 1 below): the data collection (based on available in-house data and literature review) and the toxicological evaluation.
2. Data collection
Substance information should present relevant physico-chemical data related to the test substance.
Sponsors should provide to the evaluator any internal monographs or studies of interest describing the non-clinical and clinical profile of the test substance. This documentation will support the narrative summaries addressing:
– The pharmaco-therapeutic category of concern and the mechanism of action (pharmacological properties for the API).
– The clinical use, posology and known adverse effects of the API in target and susceptible patient populations; any occupational exposure safety data or adverse effects related to other use (food, cosmetic …) of a substance may also be of interest, in particular for excipients and solvents.
– The pharmacokinetic behaviour of the test substance in human and in target veterinary or laboratory animals: this will serve the route to route and short-term to long term exposure extrapolations.
– The non-clinical (animal) data corresponding to existing toxicological investigations. These investigations should cover the 6 main endpoints of a toxicological profile:
- Acute toxicity (signs of intoxication at high dose; LD50 values).
- Local toxicity (primarily skin and eye tolerance and skin sensitization but other local effects such as gastrointestinal tract tolerance, tolerance to injectables or lung local effects through inhalation may be of concern).
- Repeated-dose toxicity: for an API, the data set will contain short-term (up to 2 weeks), subchronic (up to 3 months) and chronic (6 months to 1 year or higher durations) investigations in rodent and non-rodent species.
- Reproductive/Developmental toxicity: this section will contain studies of the impact of the test substance on male and female fertility, on embryo-foetal toxicity upon maternal exposure during pregnancy and on possible effects at parturition and during lactation and weaning of the pups.
- Genotoxicity: results of in vitro and in vivo testing related to the mutagenic and clastogenic potential of the test substance are summarised in this section.
- Carcinogenicity: dedicated animal bioassays, conducted in rodents, during their life span with records of the occurrence and development of tumours.
For solvents and excipients and for API with limited set of data on file with the Sponsor, a complementary literature search should be conducted to make sure that all key safety data are captured in the PDE monograph.
Database search should cover regulatory databases: WHO, human and veterinary health agencies databases, international and national occupational exposure limits, food, chemicals, biocides, or cosmetics databases, safety databases (e.g., HSDB, IARC) and search-engines such as PubMed.
3. Toxicological evaluation
Point of departure (PoD)
The primary step of a PDE calculation is the definition of a Point of Departure (PoD): a dose-level determined to correspond to the most critical endpoint of the overall safety profile of the test substance, obtained from the animal and human data sets.
The PoD can be selected:
– From a toxicology study with a resulting:
- LOEL or LO(A)EL i.e. Lowest Observed (Adverse) Effect Level which is the lowest dose at which there was an observed toxic or adverse effect or
- NOEL or NO(A)EL i.e. No Observed (Adverse) Effect Level which is the highest dose at which there was no observed toxic or adverse effect or
- TD50 (Toxic Dose 50%) or BMDL10 (Benchmark Dose Modelling 10%) for substances with carcinogenic potential (EFSA 2005)
– From clinical use data defining:
- an animal minimal therapeutic dose: LOEL for pharmacological effects, the most sensitive veterinarian species being considered,
- a human therapeutic dose: LOEL for pharmacological effects, the most sensitive patient’s population being considered.
– From published HBELs:
- Toxicological, microbiological, pharmacological ADI (Acceptable Daily Intakes) defined from food, agro-chemical, veterinary regulations or toxicological DNEL (Derived No Effect levels) from chemical regulation (Bercu et al; Lovsin 2017; ICH Q3D(R1); EFSA 2005).
Adjustment factors
The PDE is derived by dividing the Point of Departure (PoD) by various adjustment factors (also referred to as “safety factors”, “uncertainty factors”, “assessment factors” or “modifying factors”) to account for various uncertainties and to allow extrapolation to a reliable and robust no-effect level in the human or target animal population (EMA 2012; FDA 2005; ICHQ3C (R7); ECHA R8; Sargent 2013 ; WHO IPCS 2005).
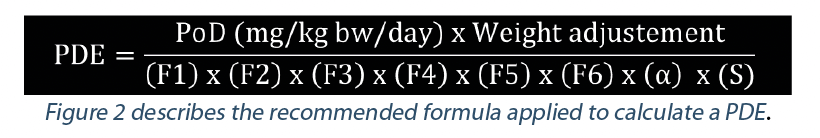
In this formula, the PDE is expressed as mg/day. According to EMA guideline (2012), a standard body weight of 50 kg for adults should be used for human medicinal products.
When the PDE has to be calculated for a paediatric population,standard body weights for new-borns (3.5 kg) or children (10 kg or 20 kg) should apply.
Another way to express the PDE is in mg/kg bw/day. This is the preferred way for veterinarian drug products. In this case the weight adjustment is not applied.
F1: Inter-species variability. F1 takes into account the comparative surface area/body weight ratios for the species concerned and for man. For example, to extrapolate from rats to human, F1=5.
F2: Inter-individual variability. EMA guideline states: “A default factor of 10, conventionally used to allow for differences between individuals in the population, is recommended.” This factor has two components, a toxicodynamic factor of 3.16 multiplied by a toxicokinetic factor of 3.16.
F3: Sub-chronic to chronic extrapolation. According to the duration of exposure related to the PoD an F3 of 1 (chronic studies) to 10 (for short-term studies) can be applied for the calculation.
F4: Modifying factor. A F4 Factor (between 1 and 10) may be applied in cases of severe toxicity, e.g. non-genotoxic carcinogenicity, neurotoxicity or teratogenicity. This should be based on the expert advice.
F5: LO(A)EL to NO(A)EL extrapolation. When only a LO(A)EL is available instead of a NO(A)EL, a factor of up to 10 can be used depending on the severity of the effect observed.
F6: Database completeness. The value of 1 to 10 attributed to this F6 factor relies on the expert judgement about the quality and completeness of the safety database for the substance of consideration.
α: Bioavailability factor. It may be necessary to extrapolate data from one route of exposure to another when estimating a PDE if the Point of Departure (PoD) is from a study conducted by a route of administration different from the route of potential exposure. The most accurate way for appraising route to route extrapolation would be building a PBPK model (Physiologically Based PharmacoKinetic modeling software). However, the time and cost for building such model preclude the use of such a tool when completing a PDE monograph.
Reichard et al. 2016 propose the estimate in Figure 3.

In the absence of data and/or where data are available but not considered sufficient, the following default bioavailability values can apply (ECHA R8, Reichard et al. 2016, Sargent et al. 2013, SCCS 2021):
- Oral bioavailability could be ranked as very low (< 1%), low (between 1 and 50%), high (between 50 and 90%) and very high (> 90%),
- Inhalation default bioavailability could be set at 100%.
- Parenteral (intravenous, subcutaneous, intramuscular …) default bioavailability could be set at 100%.
- Dermal bioavailability could be set at 10% when physicochemical parameters indicate a very low skin penetration potential, otherwise a default 50% value is applied.
However, it is always preferable using actual and reliable pharmacokinetic data when available for a compound, rather than applying a default correction factor.
S: Steady State factor. When it is estimated that the PoD originates from a study of short-term duration and with consideration of its t1/2 of plasma elimination, a bioaccumulation should be accounted when extrapolating to a chronic exposure situation., An estimate of the accumulation ratio S could be made using the equation above and applied as additional adjustment factor in the PDE calculation (Reichard et al. 2016, Sargent et al. 2013):
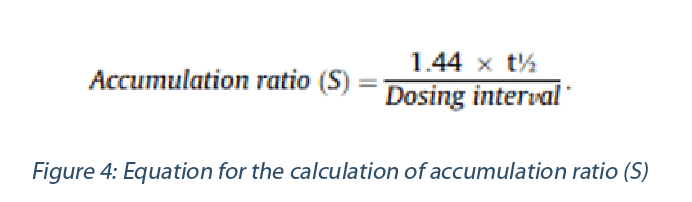
If the PoD is established from studies of sufficient duration (e.g., chronic exposure) so that steady state plasma levels have been achieved (or when circulating plasma levels are minimal), no adjustment is necessary and consequently S = 1.
In summary, the following adjustment factors should be considered when determining a PDE value (Figure 5).
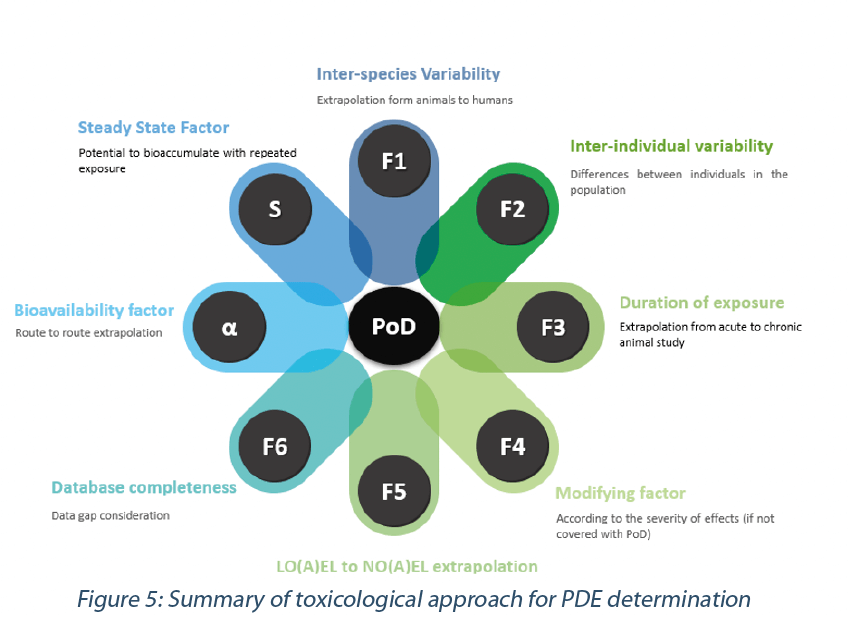
To note, it is important for the determination of the PDE:
- not to double count the same uncertainty with multiple adjustments factors,
- keep in mind that a reliable composite factor (F1xF2xF3xF4xF5xF6xαxS) should be below 5000 (Sussman et al. 2016).
4. TTC Approach in case of incomplete dataset
According to the stages achieved in drug Research and Development, pre-clinical data may be insufficient. In this case, the methodology previously described to determine a substance specific PDE cannot be used. The TTC (Threshold of Toxicological Concern) approach is a screening and prioritization tool for the safety assessment of chemicals when hazard data are incomplete (ICH M7 (R1), EFSA 2019, EMA 2012, Dolan 2005).
In this approach, threshold values expressed in μg/day are chosen according to the properties of the substances and available data: for example, a default PDE of 1.5 μg/day may be chosen for a possible mutagenic drug substance, 10 μg/day for a highly potent substance and 100 μg/day for a presumed low active substance (according to the pharmacological or toxic effect).
This approach could be accompanied by a QSAR (Quantitative structure-activity relationship) analysis to determine if the endpoint of concern would place the substance in mutagens or carcinogens or high potent category (Cramer Class III).
A large panel of QSARs is available for screening the toxicological endpoints of a test substance, including rule-based systems or statistical-based systems. These QSARs should be selected and run with the help of experts in this field.
5. Table of Hazards Identified
Once completing the safety profile of the substance, it is possible to build the Hazard identification table (Table 1) as requested by the EMA guideline.
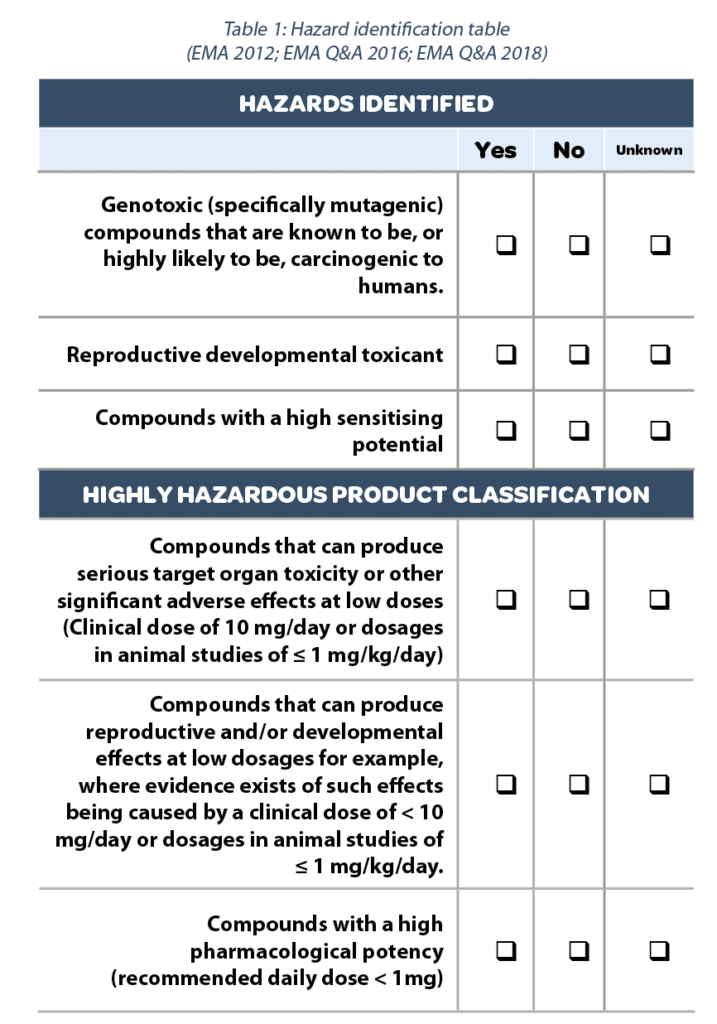
This table evidences at a glance what priority should be given to the substance. A High potent substance will drive the risk assessment in the cleaning validation process.
A highly hazardous material would correspond to a PDE value equal or below 10 μg/day, in the following scale. (Figure 6)
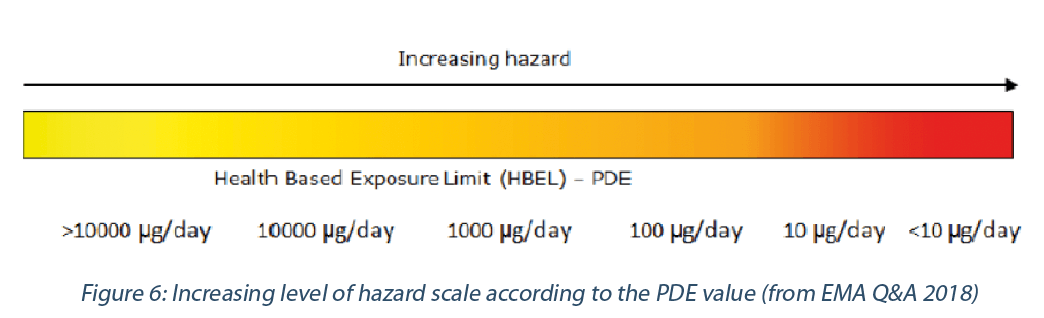
For example, hormones, cytotoxic/cytostatic agents for cancer therapies, vitamins (e.g., D) and most corticosteroids are considered as high potent APIs.
Antibiotics are not necessarily high potent agents in terms of toxicity, but their microbiological potency may decrease dramatically the PDE value which is based on a microbiological ADI.
In addition, penicillins, beta-lactams and cephalosporines are identified as highly sensitizing substances, able to induce very serious anaphylactic reactions in exposed patients. These substances require dedicated manufacturing facilities according to the GMPs (Good Manufacturing Practices) (ANSM, 2016).
6. Importance of expertise
HBEL determination should be conducted by a qualified expert, i.e, with the adequate educational background and years of experience in the practice of toxicology.
The qualified expert should be able to:
- define the right literature search,
- choose the right approach and fill the data gaps as much
as possible according to the compound (using read-across, mechanism of action…), - choose a reliable PoD,
- apply adjustments factors accordingly to address potential data gaps.
The responsibility of the qualified expert is engaged when establishing HBELs.
7. Periodic revision of the PDEs
The PDE monograph should be revised periodically, depending on the level of knowledge on the substance.
EMA Q&A 2018 specifies “HBEL, should be regularly reviewed taking account of any new relevant data”.
For solvents, excipients, and APIs with long history of use, a 5-year periodic review might be acceptable, with a simple literature search checking that no new key safety findings are published.
For APIs recently marketed, it is advised to connect to your pharmacovigilance department and ask them to alert when any new and serious safety event arises with the API. Otherwise, a check every 3 years might be appropriate.
For APIs at earlier stages of development, it is important that the safety profile be reviewed each time a pivotal safety result is obtained. In particular, the switch of the substance from Phase 2 to Phase 3 stage and then to Phase 3 stage to Marketing stage are key periods to review the PDE monographs.
Partager l’article

Maryse CORROLLER, Soline DION & Stéphane PIERRE-CEHTRA
soline.dion@cehtra.com
Glossary of abbrevations
ADE : Acceptable Daily Exposure
ADI : Acceptable Daily Intake: amount of a specific substance (originally applied for a food additive, later also for a residue of a veterinary drug or pesticide) in food or drinking water that can be ingested (orally) daily over a lifetime without an appreciable health risk.
APIs : Active Pharmaceutical Ingredients
BMDL10 : Benchmark Dose lower confidence limit 10%. The BMD is based on a mathematical model being fitted to the experimental data within the observable range and estimates the dose that causes a low but measurable response typically chosen at a 5 or 10% incidence above the control. The BMDL10 refers to
the corresponding lower limits of a one sided 95% confidence interval on the BMD.
DNEL : Derived No Effect levels
ECHA : European Chemicals Agency
EMA : European Medicines Agency
FDA : Food and Drug Administration (US)
GMPs : Good Manufacturing Practices
HBELs : Health-Based Exposure Limits
HSDB : Hazardous Substances Data Bank
IARC : International Agency for Research on Cancer
ICH : International Council for Harmonisation
LD50% : Lethal Dose 50%, amount of a substance, given once, which causes the death of 50% of a group of tested animals. The LD50 is one way to measure acute toxicity of a substance.
LO(A)EL : Lowest Observed (Adverse) Effect Level
MACO : Maximum Allowable Carry Over
NO(A)EL : No Observed (Adverse) Effect Level OEB : Occupational Exposure Band
OEL : Occupational Exposure Limit
PBPK : Physiologically Based Pharmacokinetic PDE : Permitted Daily Exposure
PoD : Point of Departure
QSAR : Quantitative structure-activity relationship REACH : Registration, Evaluation, Authorisation and Restriction of Chemicals
TD50 : Toxic Dose 50, is that chronic dose rate (in mg/kg bw per day) which would cause tumours in half of the animals within some standard experimental time – the “standard lifespan” for the species.
TDI : Tolerable Daily Intake (see ADI definition)
TTC : Threshold of Toxicological Concern
Références
• ANSM Guide des Bonne Pratiques de Fabrication. Décision du Directeur général de l’ANSM du 29 décembre 2015, modifiée par la décision du 30 décembre 2016.
• Bercu J, Morinella E, Sehner C, Shippe BK and Weideman PA. (2016). Point of departure (PoD) selection for the derivation of Acceptable Daily Exposures (ADEs) for active pharmaceutical ingredients (APIs) Regulatory Toxicology and Pharmacology 79: S48-S56.
• Dolan DG, Naumann BD, Sargent EV, Maier A, Dourson M (2005). Application of the threshold of toxicological concern concept to pharmaceutical manufacturing operations. Regul Toxicol Pharmacol, 43, 1-9.
• ECHA R8 Guidance on information requirements and chemical safety assessment. Chapter R8: Characterisation of dose [concentration]-response for human health. Version 2.1 November 2012.
• EFSA (2019) Guidance on the use of the threshold of toxicological concern approach in food safety assessment. The EFSA Journal; 17(6): 5708 (17 pages).
• EFSA (2005) Opinion of the Scientific Committee on a request from EFSA related to harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic (Request No EFSA-Q-2004-020). The EFSA Journal 280: 1-31.
• EMA guideline on HBELs (Health-Based Exposure Limits) [EMA/CHMP/ CVMP/ SWP/169430/2012]
“Guideline on setting health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities”
• Graham JC, Hillegass J, Schulze G (2020). Considerations for setting occupational exposure limits for novel pharmaceutical modalities. Regul Toxicol Pharmacol. 118 :104813.
• ICH M7 (R1): Assessment of control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk, Step 4 May 2017.
• ICH Q3C (R4) now ICH Q3C (R6) Impurities: Guideline for Residual solvents, Step 4 October 20, 2016.
• ICH Q3C (R7) Impurities: Guideline for residual solvents. Current Step 4 version dated 15 October 2018.
• ICH Q3D R1: Guideline for elemental impurities, Final version Adopted on 22 March 2019.
• Lovsin Barle E, Jandard C, Schwind M, Tuschi G, Sehner C, and Dolan DG. (2017) Comparison of Permitted Daily Exposure with 0.001 Minimal Daily Dose for Cleaning Validation. Pharmaceutical technology, 41(5): 42-53.
• Q&A: EMA/CHMP/CVMP/SWP/246844/2018
• Reichard JF, Maier MA, Naumann BD, Pecquet AM, Pfister T, Sandhu R, Sargent EV, Streeter AJ, Weideman PA (2016). Toxicokinetic and toxicodynamic considerations when deriving health-based exposure limits for pharmaceuticals. Regul Toxicol Pharmacol. 79 Suppl 1:S67-78.
• Sargent EV, Faria E, Pfister T, Sussman RG (2013). Guidance on the establishment
of acceptable daily exposure limits (ADE) to support Risk-Based Manufacture of Pharmaceutical Products. Regul Toxicol Pharmacol. 65(2):242-50.
• SCCS (Scientific Committee on Consumer Safety). The SCCS notes of guidance
for the testing of cosmetic ingredients and their safety evaluation. 11th revision. SCCS/1628/21. March 2021
• Sussman RG, Naumann BD, Pfister T, Sehner C, Seaman C, Weideman PA (2016).
A harmonization effort for acceptable daily exposure derivation – Considerations for application of adjustment factors. Regul Toxicol Pharmacol. 79 Suppl 1:S57-66.
• US FDA Guidance for Industry, Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers, July 2005.
• VICH GL18(R): Impurities: Residual solvents in new veterinary medicinal products, active substances and excipients (Revision), 19 September 2011, EMA/CVMP/ VICH/502/99-Rev.1
• WHO IPCS. Harmonization Project Document No 2. Chemical-specific adjustment factors for interspecies differences and human variability: guidance document for use of data in dose/concentration–response assessment. WHO Geneva 2005.


